Abstract
Radical resection is the only potential cure for patients with locally advanced primary and recurrent rectal cancer and is considered curative only when the histologic margins are clear of tumour. Early diagnosis of the disease is essential as it increases the likelihood of a potentially curative resection and prevention of dissemination. Clinical examination, tumour markers and radiologic modalities such as ultrasonography, computed tomography, magnetic resonance imaging and positron emission tomography are routinely used in an effort to accurately stage these patients and provide useful information for the selection of patients for further treatment/management. This review describes the methods of staging patients with locally advanced primary and recurrent rectal cancer prior to surgery emphasizing the role that radiologists have in this process.
Keywords: Staging, advanced, recurrent, rectal cancer
Introduction
A number of radiologic modalities are used for the preoperative staging of patients diagnosed with recurrent colorectal cancer within the pelvis in an effort to better select patients for surgery and medical therapy. This demonstrates the active role that radiologists have in this selection. Approximately 11,000–14,000 patients are diagnosed with rectal cancer each year in the United Kingdom[1] of which up to 16% have locally advanced tumours[2–4]. Local recurrence is diagnosed in about 15% of patients following surgery[5]. Radical resection is the only potential cure for patients with locally advanced primary and recurrent rectal cancer and is considered curative only when the histologic margins are clear of tumour (R0; 10–67% of cases)[6]. Complete tumour resection (R0) can result in 5-year survival rates of over 35%[7]. However, curative resection is only feasible when there is no dissemination of the disease and the tumour invasion at the adjacent structures is within the limits of resectability.
Therefore early diagnosis of the recurrence is crucial as it increases the likelihood of curative (R0) resection and prevention of dissemination. Clinical examination, tumour markers and radiologic modalities such as ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) are routinely used in follow-up, facilitating this and at the same time helping with the staging and planning of further treatment/management.
This review describes the methods of staging patients with recurrent pelvic colorectal cancer prior to surgery, and demonstrates the input that radiologist have in the management of these patients. The different radiologic modalities are also reviewed emphasizing their strengths and weaknesses.
Methods
A literature search was performed using Medline, Embase, Ovid and Cochrane databases for studies between 1980 and 2010 assessing radiologic modalities used to stage locally advanced primary and recurrent rectal cancer. References from each article were also reviewed. The search was limited to English language articles. The following keywords and their combination were used: “locally advanced”, “recurrent”, “rectal cancer”, “local recurrence”, “distant recurrence”, distant metastases”, “staging”, “pre-operative”, “MRI”, “CT”, “PET”, “PET/CT”, “lymph nodes”. The date of the most recent search was January 28, 2010.
Presentation
Local and distant recurrences are usually diagnosed within 2 years following surgery[8–10], during routine follow-up investigations. Patients are mainly asymptomatic but may present with symptoms related to the site of the recurrence. The symptoms may be unclear and non-specific. Extensive locoregional tumour invasion may present with pain indicating possible nerve involvement and poor prognosis.
Clinical assessment
The rectum is clinically assessed by digital examination and endoscopy in primary cancers and in recurrent cancers if bowel continuity has been maintained. Digital examination may identify luminal or extra-luminal recurrence and might provide information on relative fixation to adjacent structures[11]. Endoscopy may identify intra-luminal primary or recurrent disease and enable biopsy of the lesion. It is essential that a full colonoscopy is performed to ensure the absence of any possible synchronous lesions. A hysteroscopy and/or cystoscopy may be required to establish possible tumour invasion to the anterior structures and provide biopsies if necessary. Abdominal examination may reveal ascites, hepatomegaly, enlarged lymph nodes (Sister Mary Joseph nodule) suggestive of advanced disease.
Local staging
Accurate local staging is critical for the surgeon, as it can provide information on the extent of the disease, the type of surgery that would be required and the likelihood of complete tumour resection. Endoanal ultrasonography (EUS) has been used to diagnose recurrent disease with adequate sensitivity and specificity[12,13] and at the same time facilitates percutaneous biopsies. A meta-analysis demonstrated that EUS has 95% sensitivity and 98% specificity in staging advanced (T4) rectal cancer[14]. However, it provides limited information on the extent of the disease and cannot safely assess tumour resectability. US has limited field of view and cannot be performed when there is significant stenosis caused by intra-luminal tumour or extra-luminal pressure by tumour[15]. It has limited value following abdominoperineal excision of the rectum (APER) but can be used transvaginally in female patients to assess tumour invasion in the anterior structures.
CT is the most commonly used radiologic modality for staging primary and recurrent rectal cancer (Fig. 1a). CT has been demonstrated to have sensitivity up to 95% in detecting local recurrence[16,17]. However, it may often have difficulty distinguishing disease recurrence from tissue fibrosis[18,19] and has the tendency to overstage bladder involvement[20]. This becomes even more difficult if radiotherapy had previously been applied or there was previous pelvic sepsis from an anastomotic dehiscence[21]. Blomqvist et al.[22] demonstrated that the sensitivity of CT was low in diagnosing tumour invasion within the anterior structures (bladder and uterus; 50%) and locoregional lymph nodes (33%). The sensitivity was further reduced to 25% and increased to 50% following radiotherapy.
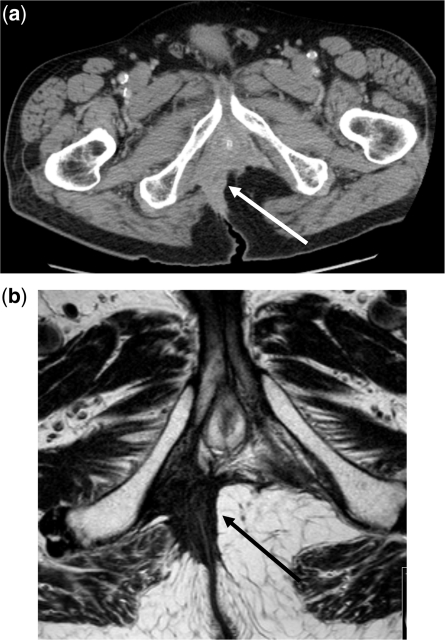
Figure 1.
Inferior compartment tumour invasion. (a) CT scan of low rectum. The arrow demonstrates the tumour. (b) MRI T2-weighted signal. The arrow demonstrates the tumour.
MRI (Fig. 1b) is very valuable when staging locally advanced primary and recurrent disease providing significant anatomic details that enable the planning of neoadjuvant therapy and surgery[23,24]. Messiou et al.[25] demonstrated that MRI was highly accurate in diagnosing tumour invasion into individual anatomic structures adjacent to the rectum but proved to be problematic when assessing the pelvic sidewalls (sensitivity = 70%) and the female reproductive organs (specificity = 33%). Compared with CT, MRI can more accurately differentiate recurrent cancer within a presacral scar (Fig. 2), based on differences in signal intensity between tumour and fibrosis using T2-weighted sequences or contrast-enhanced imaging techniques[26].
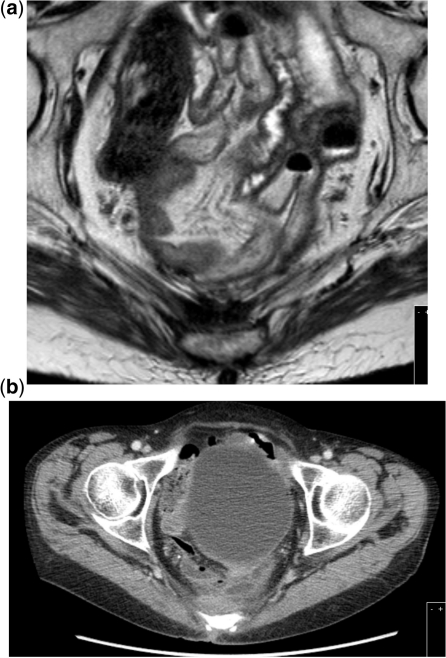
Figure 2.
Posterior tumour invasion of the sacrum. (a) MRI T2-weighted signal. (b) CT scan.
PET is an accurate diagnostic tool and may have advantages over CT and MRI in discriminating fibrosis from cancer (Fig. 3a)[27]. Exploiting the enhanced uptake of fluorodeoxyglucose (FDG) by tumour cells, PET is able to detect both local recurrence and distant metastases. A recent meta-analysis demonstrated a PET sensitivity and specificity of 94% for detecting local recurrences[28] with high accuracy in detecting pelvic recurrence in patients who had previously been irradiated[29]. However, limitations of PET scan include the inability to identify small-volume disease and a relatively low sensitivity for detecting lymph node metastases[30]. Mucinous adenocarcinomas have poorer FDG uptake and therefore can be easily missed on PET scan[31]. In an effort to increase the confidence in diagnosing recurrence, PET with CT (PET/CT) image fusion was performed. Sapir et al.[32] investigated the role of PET/CT in 62 patients and demonstrated that PET/CT was more accurate than PET alone for detecting local recurrence but is not very helpful in evaluating anatomic tumour changes following chemoradiotherapy[33]. It might be useful in predicting pathologic tumour response however (Fig. 3b)[33–35].
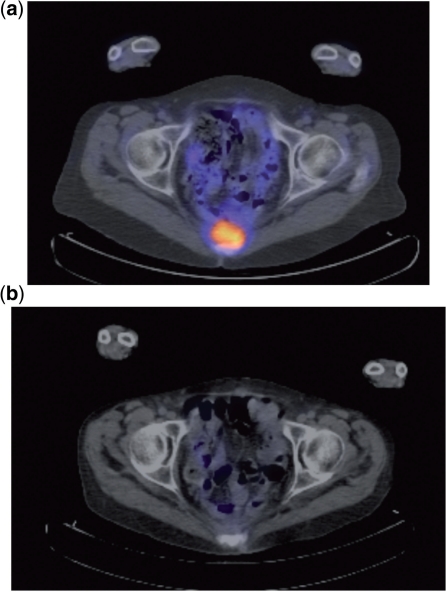
Figure 3.
Posterior tumour recurrence invading the sacrum. (a) PET demonstrating increased FDG uptake from the tumour before neoadjuvant chemoradiotherapy. (b) PET demonstrating reduced FDG uptake from the tumour following neoadjuvant chemoradiotherapy.
Classifications for local disease
In an effort to establish criteria that would enable better prediction of tumour resectability and outcome, a number of classifications have been proposed (Table 1). The Mayo Clinic classified local recurrence according to points of tumour fixation and pathologic findings[36] while Yamada et al.[37] described local recurrence by pattern of pelvic fixation into localized, sacral, or lateral. Other proposed classification systems[38] are based on the UICC TNM system[39]. The Memorial Sloan Kettering group have categorized local recurrence based on the anatomic site of invasion by the tumour[40–42]. A new classification has been also proposed based on the fascial boundaries and planes of dissection between intra-pelvic organs[43].
Table 1.
Classification systems to predict tumour resectability
| Study group | Classification | Definitions | Outcomes | |
|---|---|---|---|---|
| Mayo Clinic[36] | Symptoms | S0 | Asymptomatic | Patients who were pain-free had better survival |
| S1 | Symptomatic without pain | |||
| S2 | Symptomatic with pain | |||
| Degree and site of Fixation | F0 | No fixation | More points of fixation resulted in more complications and worse survival | |
| F1 | Fixation to 1 point | |||
| F2 | Fixation to 2 points | |||
| F3 | Fixation to >2 points | |||
| Yamada[37] | Pattern of pelvic fixation | Localized | Invasion to adjacent pelvic organs or tissue | 5-year survival of 38% |
| Sacral invasive | Invasion to lower sacrum (S3, S4, S5), coccyx, periosteum | 5-year survival of 10% | ||
| Lateral invasive | Invasion to sciatic nerve, greater sciatic foramen, lateral pelvic wall, upper sacrum (S1, S2) | 5-year survival of 0% | ||
| Wanebo[38] | Five stages | TR1 | Limited invasion of the muscularis | |
| TR2 | Full thickness penetration of muscularis propria | |||
| TR3 | Anastomotic recurrences with full thickness penetration beyond the bowel wall and into the perirectal soft tissue | |||
| TR4 | Invasion into adjacent organs without fixation | |||
| TR5 | Invasion of the bony ligamentous pelvis including sacrum, low pelvic/side walls, or sacrotuberous/ischial ligaments | |||
| MSK[40–42] | Anatomic region involved | Axial | Anastomotic, mesorectal, perirectal soft tissue, perineum (APER) | Axial only recurrence has 90% likelihood of R0; lateral recurrence is associated with 36% likelihood of R0 |
| Anterior | Genitourinary tract | |||
| Posterior | Sacrum and presacral fascia | |||
| Lateral | Soft tissues of the pelvic sidewall and lateral bony pelvis | |||
| RMH[43] | MRI; Planes of dissection | Central | Rectum or neo-rectum, intra-luminal recurrence, peri-rectal fat or mesorectum, extra-luminal recurrence | MRI diagnosis of tumour invasion within the lateral, posterior or in more than 2 compartments was associated with reduced disease-free survival |
| PR | Rectovesical pouch or recto-uterine pouch of Douglas | |||
| AA PR | Ureters and iliac vessels above the peritoneal reflection, sigmoid colon, small bowel and lateral side wall fascia | |||
| AB PR | Genitourinary system | |||
| Lateral | Ureters, external and internal iliac vessels, lateral pelvic lymph nodes, sciatic nerve, sciatic notch, S1 and S2 nerve roots, piriformis or obturator internus muscle | |||
| Posterior | Coccyx, presacral fascia, retro-sacral space, sacrum up to the upper level of S1 | |||
| Inferior | Levator ani muscles, external sphincter complex, perineal scar (APER), ischio-anal fossa |
The Mayo Clinic[36] classified recurrences according to symptoms and the degree and site of fixation. The patients who presented without pain had better 3-year (68.4% vs 31.6%) and 5-year (37.3% vs 26.3%) survival compared with the patients who were symptomatic with pain (p = 0.065). They demonstrated that patients with more points of fixation (F1–3) had more complications following surgery and had worse 3-year (35.7% vs 61.3%) and 5-year (31.2% vs 50%) survival compared with the patients with an F0 stage (p = 0.384).
Yamada et al.[37] classified local recurrence according to the pattern of pelvic fixation. It was demonstrated that the pattern of recurrence was a predictive factor for 5-year survival following surgery (p < 0.001). The 5-year survival rate was 38%, 10% and 0% for patients with localized, sacral invasive and lateral invasive type of recurrence, respectively.
Wanebo et al.[38] proposed a five-stage classification system describing intra-luminal local recurrence at the anastomotic site as TR 1 or 2 corresponding to limited invasion of the muscularis and full thickness penetration of muscularis propria, respectively. TR3 described anastomotic recurrences with full thickness penetration beyond the bowel wall and into the perirectal soft tissue. Invasion into adjacent structures/organs including the vagina, uterus, prostate, bladder, seminal vesicles or presacral tissues with tethering but not fixation was described as TR4. Invasion of the bony ligamentous pelvis including the sacrum, low pelvic/side walls, or sacrotuberous/ischial ligaments was described as TR5.
The Memorial Sloan Kettering (MSK) group[40–42] have described a classification based on the anatomic region of the pelvis that is involved. Local recurrence was defined as either axial, anterior, posterior or lateral. Axial recurrence was subdivided into anastomotic, mesorectal or perirectal soft tissue, or perineum if an APER was previously performed. Moore et al.[41] from the MSK group demonstrated that with axial only or axial/anterior only recurrences, the likelihood for a complete resection was high (90% vs 43%, p < 0.001 and 72% vs 42%, p = 0.003 respectively). The likelihood for a complete resection was significantly reduced however when there was a lateral recurrence (36% vs 65%, p = 0.002).
More recently, the authors have proposed a new classification based on the fascial boundaries and the anatomic planes of dissection between intra-pelvic organs[43]. The recurrence is classified based on the MRI findings of tumour invasion within 7 intra-pelvic compartments (Table 1): the central, peritoneal reflection, anterior above and below the peritoneal reflection, posterior, lateral and inferior compartments. This is currently the classification adopted at the authors' institute to stage this group of patients. More details are shown in Table 1.
All the classifications aim to describe the recurrent tumour is fixed to and hence establish the potential for and extent of resection in order to define selection criteria for surgery along with prognostic information. The authors have found the latter to be the most useful classification enabling better communication and understanding between radiologists and clinicians, patient selection for surgery and surgical planning, providing at the same time detailed information on the extent of the disease and signifying prognosis.
Imaging for distant metastases
Accurate identification of extrapelvic disease is key for the decision to operate on a patient. CT and MRI have demonstrated high sensitivity in detecting distant recurrence providing detailed anatomic information on the affected organ and tumour extension into surrounding tissues[20,44]. The accuracy of CT in detecting abdominal disease has been demonstrated to be over 85%[20] and MRI has similar accuracy[23,24].
A recent meta-analysis investigating the value of US, CT, MRI and PET in detecting liver metastases (Fig. 4) demonstrated sensitivity of 63%, 74.8%, 81.1% and 97.2%, respectively, and specificity of more than 93.8%, with MRI being significantly more sensitive than CT (p = 0.05) and equally sensitive to PET (p = 0.02)[44]. There was no significant difference in the sensitivity of PET and CT (p > 0.05) or between CT and US (p = 0.45)[45].
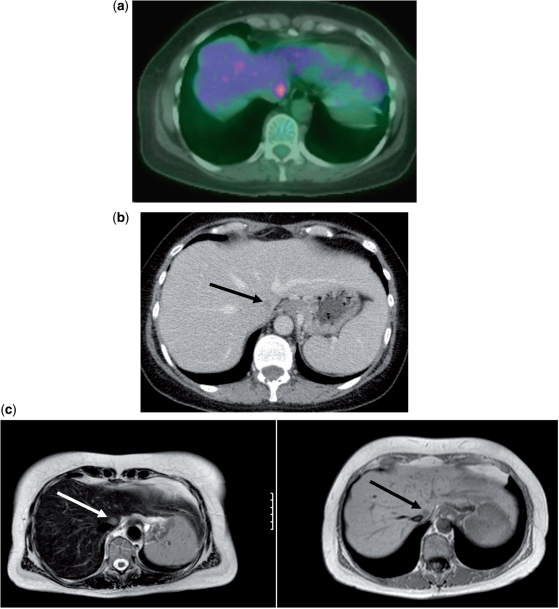
Figure 4.
A 1-cm focus of liver metastases in the caudate lobe medial to the inferior vena cava and lateral to the oesophageal hiatus. (a) PET. (b) CT scan. (c) MRI. T2-weighted image on the left and T1-weighted image on the right.
PET has been demonstrated to be highly accurate in the detection of disseminated disease[46–49] and to have significant impact on the management of patients with suspected recurrent colorectal cancer[50,51]. A meta-analysis reported PET sensitivity of 91% and specificity of 83% for the diagnosis of distant metastases[28]. However, the authors admitted that only 8/27 (29.6%) studies were of high quality, fulfilling their quality criteria by at least 80%. Another study showed that the overall added value of PET in the management of patients with local and/or distant recurrent colorectal cancer is 8% and suggested that PET should be used when findings remain equivocal after serial imaging review[52]. In the authors practice, all patients considered for a potentially curative surgical resection undergo a PET scan to exclude disseminated disease.
Selection criteria for surgery
The decision for surgery is made after extensive discussions at the local multidisciplinary meeting and relies heavily on the findings of the available diagnostic modalities. It is the radiologic findings that will determine the tumour resectability. Therefore accurate preoperative staging can help to establish the extent of local disease and the presence or absence of distant metastases, which is imperative when considering patients for exenterative surgery for locally advanced primary or recurrent rectal cancer.
Distant recurrence
The presence of distant metastases is normally considered as a contraindication for exenterative surgery for locally advanced primary or recurrent rectal cancer[53]. It has been demonstrated by some centres however that synchronous or staged resection of locoregional recurrence and distant metastases can have acceptable results in a select group of patients[54–56]. However, it is generally contraindicated due to the significant morbidity that may be associated with this type of procedure[37,38,57–59].
Resectable local recurrence
In the absence of distant disease, surgical resection of the primary cancer or the locoregional recurrence is the only potentially curative option. Surgery for advanced primary or recurrent rectal cancer includes a range of different procedures that depend on the extent of the disease and the specific organs/structures that are involved. Surgery is performed en bloc and is considered curative when the resection margins are free of microscopic disease (R0 resection). Microscopic and macroscopic residual disease at the resection margins are defined as R1 and R2 resection, respectively. It has been previously demonstrated that R1 or R2 resection can result in poorer survival[7,36,60,61] and consequently it should be considered as palliative resection with considerable morbidity. Therefore the identification of patients who can potentially achieve an R0 resection is crucial and extremely difficult. Preoperative imaging with PET, CT and MRI and clinical assessment are utilized in an effort to optimize the selection of patients in whom curative resection is considered possible as well as those in whom resection is contraindicated.
Contraindications for surgical resection
It is essential to assess the patients' fitness for surgery before discussing surgery with the patient because the lack of fitness could be a contraindication of its own when undergoing such a major procedure. Surgery is contraindicated in the presence of circumferential or extensive lateral pelvic side wall involvement, involvement of the iliac vessels, bilateral ureteric obstruction, sciatic nerve involvement and periaortic lymph node metastases[53,54,62–64]. Involvement of the iliac vessels may present with lower limb oedema whereas ureteric obstruction presents with hydronephrosis. Tumour invasion of the sciatic nerve may present with lower limb pain and weakness. Limited tumour invasion to the lateral pelvic wall and invasion of the sacrum above the S2 vertebrae are considered relative contraindications since there are surgical options in both cases[38,57,65] but the likelihood of complete resection is very low.
Irresectable local recurrence
Surgical resection and chemoradiotherapy can be used for palliation alleviating the patients' symptoms that are related to the organs/structure that are invaded by tumour. It has been suggested that palliative resection can have an improvement in quality of life and pain relief[66,67]. However, the co-morbidities related with this type of surgery must also be considered[68]. It is therefore important that the patients are carefully selected for palliative procedures taking into consideration possible co-morbidities and their social circumstances, because the benefits from these procedures are short term. Symptomatic relief can last up to 17 months with a median symptom-free interval of 4 months compared with 23 months for non-palliative procedures (p < 0.001)[69].
Discussion
The present review demonstrates the important contribution that a radiologist might have in the decision making for patients under consideration for exenterative pelvic surgery for recurrent colorectal pelvic cancer. The main objective in this process is to filter the patients who would most benefited from surgery by achieving complete tumour resection. The process of selecting the patients who should undergo surgery or best medical therapy is guided by the discussions within the multidisciplinary meetings. In these meetings, radiologists carry a key role as the ultimate decision for surgery or palliative care usually rests with them. This is why a number of radiologic modalities are used with the aim of maximizing the information provided and therefore a better informed decision for further management can be made.
The role of CT and MRI scans is to stage the extent of local disease within the pelvis. More recently, EUS has been used to identify local recurrence and the local extent of the disease. Diffusion-weighted MRI, which has also been used in recent years, may provide additional information on the extent of the disease but its role is still unclear and is not validated due to the absence of substantial evidence regarding its diagnostic accuracy. PET/CT and CT scans are used to filter patients with evidence of distant metastases. When there is doubt and controversial findings among the different radiologic modalities, laparoscopy might be used to clarify the presence of distant metastases. The use of all these modalities is evidence of the fundamental role that radiologists have in the management of patients who are considered for exenterative surgery for recurrent colorectal pelvic cancer.
Accurate preoperative staging is crucial for the management of the patients with locally advanced primary and recurrent rectal cancer. It can provide the necessary information to make decisions on tumour resectability and type of surgery required to achieve complete resection. CT, MRI and PET all have a place in the preoperative staging of these patients. In the authors' practice, MRI is used to plan surgery as it can provide anatomic details; CT and PET are mostly used to exclude distant metastases. Radiotherapy and previous surgery can reduce the accuracy of all the diagnostic modalities and this needs to be taken into consideration when interpreting scans following radiotherapy. Therefore, adequate training and experience in interpreting these images is required so that the radiologists can maximize their contribution in the management of this group of patients. Further good quality prospective studies are needed to better identify patient selection criteria for surgery, to enhance the oncologic outcome and the improve the patients' quality of life.
References
- 1.Nicholls RJ, Tekkis PP. Multidisciplinary treatment of cancer of the rectum: a European approach. Surg Oncol Clin N Am. 2008;17:533–51, viii. doi: 10.1016/j.soc.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Boey J, Wong J, Ong GB. Pelvic exenteration for locally advanced colorectal carcinoma. Ann Surg. 1982;195:513–18. doi: 10.1097/00000658-198204000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilepich MV, Munzenrider JE, Tak WK, Miller HH. Preoperative irradiation of primarily unresectable colorectal carcinoma. Cancer. 1978;42:1077–81. doi: 10.1002/1097-0142(197809)42:3<1077::AID-CNCR2820420307>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Spratt JS, Jr, Spjut HJ. Prevalence and prognosis of individual clinical and pathologic variables associated with colorectal carcinoma. Cancer. 1967;20:1976–85. doi: 10.1002/1097-0142(196711)20:11<1976::AID-CNCR2820201125>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Heriot K. Rectal cancer recurrence: factors and mechanisms. Color Dis. 2000;2:126–36. doi: 10.1046/j.1463-1318.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- 6.Asoglu O, Karanlik H, Muslumanoglu M, et al. Prognostic and predictive factors after surgical treatment for locally recurrent rectal cancer: a single institute experience. Eur J Surg Oncol. 2007;33:1199–206. doi: 10.1016/j.ejso.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Heriot AG, Byrne CM, Lee P, et al. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284–91. doi: 10.1007/s10350-007-9152-9. [DOI] [PubMed] [Google Scholar]
- 8.Rao AR, Kagan AR, Chan PM, Gilbert HA, Nussbaum H, Hintz BL. Patterns of recurrence following curative resection alone for adenocarcinoma of the rectum and sigmoid colon. Cancer. 1981;48:1492–5. doi: 10.1002/1097-0142(19810915)48:6<1492::AID-CNCR2820480636>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following ‘curative’ surgery for large bowel cancer: II. The rectum and rectosigmoid. Br J Surg. 1984;71:17–20. doi: 10.1002/bjs.1800710105. [DOI] [PubMed] [Google Scholar]
- 10.Cass AW, Million RR, Pfaff WW. Patterns of recurrence following surgery alone for adenocarcinoma of the colon and rectum. Cancer. 1976;37:2861–5. doi: 10.1002/1097-0142(197606)37:6<2861::AID-CNCR2820370643>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11. Paterson C, Nelson H. Surgical approach to locally recurrent disease. In: Audisio RA, Geraghty JG, Longo WE, editors. Modern management of cancer of the rectum. London: Springer-Verlag; 2001, p. 147–56. [Google Scholar]
- 12.Rifkin MD, Ehrlich SM, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology. 1989;170:319–22. doi: 10.1148/radiology.170.2.2643135. [DOI] [PubMed] [Google Scholar]
- 13.Beynon J, Mortensen NJ, Foy DM, Channer JL, Rigby H, Virjee J. The detection and evaluation of locally recurrent rectal cancer with rectal endosonography. Dis Colon Rectum. 1989;32:509–17. doi: 10.1007/BF02554508. [DOI] [PubMed] [Google Scholar]
- 14.Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254–65. doi: 10.1245/s10434-008-0231-5. [DOI] [PubMed] [Google Scholar]
- 15.Kruskal JB, Kane RA, Sentovich SM, Longmaid HE. Pitfalls and sources of error in staging rectal cancer with endorectal us. Radiographics. 1997;17:609–26. doi: 10.1148/radiographics.17.3.9153700. [DOI] [PubMed] [Google Scholar]
- 16.Moss AA, Thoeni RF, Schnyder P, Margulis AR. Value of computed tomography in the detection and staging of recurrent rectal carcinomas. J Comput Assist Tomogr. 1981;5:870–4. doi: 10.1097/00004728-198112000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Adalsteinsson B, Glimelius B, Graffman S, Hemmingsson A, Pahlman L, Rimsten A. Computed tomography of recurrent rectal carcinoma. Acta Radiol Diagn (Stockh) 1981;22:669–72. doi: 10.1177/028418518102200608. [DOI] [PubMed] [Google Scholar]
- 18.Grabbe E, Winkler R. Local recurrence after sphincter-saving resection for rectal and rectosigmoid carcinoma. Value of various diagnostic methods. Radiology. 1985;155:305–10. doi: 10.1148/radiology.155.2.3983380. [DOI] [PubMed] [Google Scholar]
- 19.Thompson WM, Halvorsen RA, Foster WL, Jr., Roberts L, Gibbons R. Preoperative and postoperative CT staging of rectosigmoid carcinoma. AJR Am J Roentgenol. 1986;146:703–10. doi: 10.2214/ajr.146.4.703. [DOI] [PubMed] [Google Scholar]
- 20.Farouk R, Nelson H, Radice E, Mercill S, Gunderson L. Accuracy of computed tomography in determining resectability for locally advanced primary or recurrent colorectal cancers. Am J Surg. 1998;175:283–7. doi: 10.1016/S0002-9610(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 21.Heriot AG, Tekkis PP, Darzi A, Mackay J. Surgery for local recurrence of rectal cancer. Colorectal Dis. 2006;8:733–47. doi: 10.1111/j.1463-1318.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 22.Blomqvist L, Holm T, Goranson H, Jacobsson H, Ohlsen H, Larsson SA. MR imaging, CT and CEA scintigraphy in the diagnosis of local recurrence of rectal carcinoma. Acta Radiol. 1996;37:779–84. doi: 10.3109/02841859609177716. [DOI] [PubMed] [Google Scholar]
- 23.Balzarini L, Ceglia E, D'Ippolito G, Petrillo R, Tess JD, Musumeci R. Local recurrence of rectosigmoid cancer: what about the choice of MRI for diagnosis? Gastrointest Radiol. 1990;15:338–42. doi: 10.1007/BF01888814. [DOI] [PubMed] [Google Scholar]
- 24.Pema PJ, Bennett WF, Bova JG, Warman P. CT vs MRI in diagnosis of recurrent rectosigmoid carcinoma. J Comput Assist Tomogr. 1994;18:256–61. doi: 10.1097/00004728-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Messiou C, Chalmers AG, Boyle K, Wilson D, Sagar P. Pre-operative MR assessment of recurrent rectal cancer. Br J Radiol. 2008;81:468–73. doi: 10.1259/bjr/53300246. [DOI] [PubMed] [Google Scholar]
- 26.Dicle O, Obuz F, Cakmakci H. Differentiation of recurrent rectal cancer and scarring with dynamic MR imaging. Br J Radiol. 1999;72:1155–9. doi: 10.1259/bjr.72.864.10703471. [DOI] [PubMed] [Google Scholar]
- 27.Huebner RH, Park KC, Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41:1177–89. [PubMed] [Google Scholar]
- 28.Zhang C, Chen Y, Xue H, et al. Diagnostic value of FDG-PET in recurrent colorectal carcinoma: a meta-analysis. Int J Cancer. 2009;124:167–73. doi: 10.1002/ijc.23926. [DOI] [PubMed] [Google Scholar]
- 29.Moore HG, Akhurst T, Larson SM, Minsky BD, Mazumdar M, Guillem JG. A case-controlled study of 18-fluorodeoxyglucose positron emission tomography in the detection of pelvic recurrence in previously irradiated rectal cancer patients. J Am Coll Surg. 2003;197:22–8. doi: 10.1016/S1072-7515(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 30.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405–22. doi: 10.1148/radiol.2382041977. [DOI] [PubMed] [Google Scholar]
- 31.Kamel IR, Cohade C, Neyman E, Fishman EK, Wahl RL. Incremental value of CT in PET/CT of patients with colorectal carcinoma. Abdom Imaging. 2004;29:663–8. doi: 10.1007/s00261-003-0163-2. [DOI] [PubMed] [Google Scholar]
- 32.Even-Sapir E, Parag Y, Lerman H, et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology. 2004;232:815–22. doi: 10.1148/radiol.2323031065. [DOI] [PubMed] [Google Scholar]
- 33.Vliegen RF, Beets-Tan RG, Vanhauten B, et al. Can an FDG-PET/CT predict tumor clearance of the mesorectal fascia after preoperative chemoradiation of locally advanced rectal cancer? Strahlenther Onkol. 2008;184:457–64. doi: 10.1007/s00066-008-1858-7. [DOI] [PubMed] [Google Scholar]
- 34.Kristiansen C, Loft A, Berthelsen AK, et al. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 2008;51:21–5. doi: 10.1007/s10350-007-9095-1. [DOI] [PubMed] [Google Scholar]
- 35.Capirci C, Rampin L, Erba PA, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34:1583–93. doi: 10.1007/s00259-007-0426-1. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Dozois RR, Devine RM, et al. Curative reoperations for locally recurrent rectal cancer. Dis Colon Rectum. 1996;39:730–6. doi: 10.1007/BF02054435. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001;88:988–93. doi: 10.1046/j.0007-1323.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- 38.Wanebo HJ, Antoniuk P, Koness RJ, et al. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438–48. doi: 10.1007/BF02235044. [DOI] [PubMed] [Google Scholar]
- 39.Sobin LH, Fleming ID. TNM classification of malignant tumors, fifth edition. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–4. doi: 10.1002/(SICI)1097-0142(19971101)80:9<1803::AID-CNCR16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Guillem J, Ruo L. Strategies in operative therapy for locally recurrent rectal cancer. Semin Colon Rectal Cancer. 1998;9:259–68. [Google Scholar]
- 41.Moore HG, Shoup M, Riedel E, et al. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47:1599–606. doi: 10.1007/s10350-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 42.Moore H, Harrison L, Guillem J. Cancer of the rectum: follow up and management of local recurrence. In: Fazio VW, Church JM, Delaney CP, editors. Current therapy in colon and rectal surgery. 2nd ed. Philadelphia, PA: Elsevier Mosby; 2005. pp. 185–90. [Google Scholar]
- 43.Georgiou P, Brown G, Constantinides V, Antoniou A, Nicholls RJ, Tekkis PP. Diagnostic accuracy of MRI in assessing tumor invasion within pelvic compartments in recurrent and locally advanced rectal cancer. Dis Colon Rectum. 2009;52:9. [Google Scholar]
- 44.Ward BA, Miller DL, Frank JA, et al. Prospective evaluation of hepatic imaging studies in the detection of colorectal metastases: correlation with surgical findings. Surgery. 1989;105:180–7. [PubMed] [Google Scholar]
- 45.Floriani I, Torri V, Rulli E, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–31. doi: 10.1002/jmri.22010. [DOI] [PubMed] [Google Scholar]
- 46.Falk PM, Gupta NC, Thorson AG, et al. Positron emission tomography for preoperative staging of colorectal carcinoma. Dis Colon Rectum. 1994;37:153–6. doi: 10.1007/BF02047538. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Nabi H, Doerr RJ, Lamonica DM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998;206:755–60. doi: 10.1148/radiology.206.3.9494497. [DOI] [PubMed] [Google Scholar]
- 48.Ogunbiyi OA, Flanagan FL, Dehdashti F, et al. Detection of recurrent and metastatic colorectal cancer: comparison of positron emission tomography and computed tomography. Ann Surg Oncol. 1997;4:613–20. doi: 10.1007/BF02303744. [DOI] [PubMed] [Google Scholar]
- 49.Mukai M, Sadahiro S, Yasuda S, et al. Preoperative evaluation by whole-body 18F-fluorodeoxyglucose positron emission tomography in patients with primary colorectal cancer. Oncol Rep. 2000;7:85–7. [PubMed] [Google Scholar]
- 50.Meta J, Seltzer M, Schiepers C, et al. Impact of 18F-FDG PET on managing patients with colorectal cancer: the referring physician's perspective. J Nucl Med. 2001;42:586–90. [PubMed] [Google Scholar]
- 51.Kalff V, Hicks RJ, Ware RE, Hogg A, Binns D, McKenzie AF. The clinical impact of (18)F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med. 2002;43:492–9. [PubMed] [Google Scholar]
- 52.Potter KC, Husband JE, Houghton SL, Thomas K, Brown G. Diagnostic accuracy of serial CT/magnetic resonance imaging review vs. positron emission tomography/CT in colorectal cancer patients with suspected and known recurrence. Dis Colon Rectum. 2009;52:253–9. doi: 10.1007/DCR.0b013e31819d11e6. [DOI] [PubMed] [Google Scholar]
- 53.Paterson C, Nelson H. Surgical approach to locally recurrent disease. In: Audisio R, Geraghty J, Longo W, editors. Modern management of cancer of the rectum. London: Springer-Verlag; 1998. pp. 147–56. [Google Scholar]
- 54.Huguier M, Houry S. Treatment of local recurrence of rectal cancer. Am J Surg. 1998;175:288–92. doi: 10.1016/S0002-9610(98)00016-6. [DOI] [PubMed] [Google Scholar]
- 55.Maetani S, Onodera H, Nishikawa T, et al. Significance of local recurrence of rectal cancer as a local or disseminated disease. Br J Surg V. 85:521–5. doi: 10.1046/j.1365-2168.1998.00602.x. [DOI] [PubMed] [Google Scholar]
- 56.Hartley JE, Lopez RA, Paty PB, Wong WD, Cohen AM, Guillem JG. Resection of locally recurrent colorectal cancer in the presence of distant metastases: can it be justified? Ann Surg Oncol. 2003;10:227–33. doi: 10.1245/ASO.2003.05.039. [DOI] [PubMed] [Google Scholar]
- 57.Moriya Y, Akasu T, Fujita S, Yamamoto S. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Dis Colon Rectum. 2004;47:2047–53; discussion 2053–4. doi: 10.1007/s10350-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 58.Hahnloser D, Nelson H, Gunderson LL, et al. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg. 2003;237:502–8. doi: 10.1097/00000658-200304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Aguilar J, Cromwell JW, Marra C, Lee SH, Madoff RD, Rothenberger DA. Treatment of locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:1743–8. doi: 10.1007/BF02234449. [DOI] [PubMed] [Google Scholar]
- 60.Sagar PM, Gonsalves S, Heath RM, Phillips N, Chalmers AG. Composite abdominosacral resection for recurrent rectal cancer. Br J Surg. 2009;96:191–6. doi: 10.1002/bjs.6464. [DOI] [PubMed] [Google Scholar]
- 61.Shoup M, Guillem JG, Alektiar KM, et al. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum. 2002;45:585–92. doi: 10.1007/s10350-004-6250-9. [DOI] [PubMed] [Google Scholar]
- 62.Yeung RS, Moffat FL, Falk RE. Pelvic exenteration for recurrent colorectal carcinoma: a review. Cancer Invest. 1994;12:176–88. doi: 10.3109/07357909409024873. [DOI] [PubMed] [Google Scholar]
- 63.Cheng C, Rodriguez-Bigas MA, Petrelli N. Is there a role for curative surgery for pelvic recurrence from rectal carcinoma in the presence of hydronephrosis? Am J Surg. 2001;182:274–7. doi: 10.1016/S0002-9610(01)00706-1. [DOI] [PubMed] [Google Scholar]
- 64.Mannaerts GH, Rutten HJ, Martijn H, Groen GJ, Hanssens PE, Wiggers T. Abdominosacral resection for primary irresectable and locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:806–14. doi: 10.1007/BF02234699. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki K, Gunderson LL, Devine RM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939–52. doi: 10.1002/1097-0142(19950215)75:4<939::AID-CNCR2820750408>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 66.Yeung RS, Moffat FL, Falk RE. Pelvic exenteration for recurrent and extensive primary colorectal adenocarcinoma. Cancer. 1993;72:1853–8. doi: 10.1002/1097-0142(19930915)72:6<1853::AID-CNCR2820720611>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 67.Brophy PF, Hoffman JP, Eisenberg BL. The role of palliative pelvic exenteration. Am J Surg. 1994;167:386–90. doi: 10.1016/0002-9610(94)90121-X. [DOI] [PubMed] [Google Scholar]
- 68.Belluco C, Melega E, Mammano E, Pucciarelli S, Nitti D, Lise M. Multimodality management of recurrent rectal cancer. Clin Colon Rectal Surg. 2002;15:63. doi: 10.1055/s-2002-23569. [DOI] [Google Scholar]
- 69.Miner TJ, Jaques DP, Paty PB, Guillem JG, Wong WD. Symptom control in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2003;10:72–9. doi: 10.1245/ASO.2003.03.040. [DOI] [PubMed] [Google Scholar]