Abstract
Background
Schizophrenia patients demonstrate impairment on visual backward masking, a measure of early visual processing. Most visual masking paradigms involve two distinct processes, an early fast-acting component associated with object formation and a later component that acts through object substitution. So far, masking paradigms used in schizophrenia research have been unable to separate these two processes.
Method
We administered three visual processing paradigms (location masking with forward and backward masking, four-dot backward masking and a cuing task) to 136 patients with schizophrenia or schizoaffective disorder and 79 healthy controls. A psychophysical procedure was used to match subjects on identification of an unmasked target prior to location masking. Location masking interrupts object formation, four-dot masking task works through masking by object substitution and the cuing task measures iconic decay.
Results
Patients showed impairment on location masking after being matched for input threshold, similar to previous reports. After correcting for age, patients showed lower performance on four-dot masking than controls, but the groups did not differ on the cuing task.
Conclusions
Patients with schizophrenia showed lower performance when masking was specific to object substitution. The difference in object substitution masking was not due to a difference in rate of iconic decay, which was comparable in the two groups. These results suggest that, despite normal iconic decay rates, individuals with schizophrenia show impairment in a paradigm of masking by object substitution that did not also involve disruption of object formation.
Keywords: Early perception, object substitution, schizophrenia, visual masking
Introduction
In a visual masking paradigm, the visibility of a target is disrupted by the presence of a mask that occurs briefly before or after the target, for forward masking and backward masking, respectively (Breitmeyer, 1984; Breitmeyer & Ogmen, 2000). A well-replicated finding is that schizophrenia patients perform more poorly than comparison subjects in identifying a visual target in the presence of a mask (Saccuzzo & Braff, 1981, 1986; Saccuzzo & Schubert, 1981; Rund, 1993; Green et al. 1994a, b; Cadenhead et al. 1998).
Visual masking in schizophrenia has been studied for several reasons, including evidence that impairment on this paradigm may indicate vulnerability to schizophrenia (Miller et al. 1979; Lieb et al. 1996; Green et al. 1997, 1999, 2006; Keri et al. 2001; Bedwell et al. 2003). Also, deficits on masking tasks are related to functional outcome through key mediating variables, such as social cognition (Sergi et al. 2006; Rassovsky et al. 2010). An additional reason for applying masking paradigms to the study of schizophrenia is that they can be used to probe underlying neural systems. For example, masking paradigms have been used to probe the relative contribution of the magnocellular versus parvocellular visual processing channels (Schuck & Lee, 1989; Green et al. 1994b; Cadenhead et al. 1998; Schechter et al. 2003), the role of neural oscillations in the γ band (Green et al. 2003a; Wynn et al. 2005) and the functioning of key visual processing regions, such as the lateral occipital complex (area LO) (Green et al. 2009).
There is strong evidence to suggest that most visual masking paradigms involve two distinct processes, an early fast-acting component associated with object formation and a later component that acts through object substitution (Di Lollo et al. 2000; Enns & Di Lollo, 2000; Ro et al. 2003; Enns, 2004; Chen & Treisman, 2009; Dux et al. 2010). Masking associated with object formation occurs in a very brief temporal window (i.e. target-mask intervals of 0–100 ms), when the target and mask are presented so closely in time that they essentially join together and form an integrated target–mask composite that makes it hard to identify the target. In contrast, masking by object substitution occurs when the target percept is replaced by the mask before it reaches awareness and this effect is maximal at delays following the first 100 ms. The theory behind masking by object substitution is that perception is a consequence of recurrent communication between lower-level and higher-level neural processes. Initially, the information is handled by lower-level units in a ‘feed forward’ sweep. Feed forward models of visual processing (i.e. those that involve the flow of information from the retina through to the hierarchical stages of visual cortex) have been predominant for explaining visual masking. However, cortical feedback (i.e. re-entrant) sweeps are necessary to resolve ambiguity between possible alternative visual patterns. Recent advances in cognitive and perceptual neuroscience have emphasized the importance of these re-entrant processes of feedback from higher to lower levels of the cortical hierarchy for processing visual stimuli within the cortex. These re-entrant processes appear to be essential for conscious perception (Ro et al. 2003; Chen & Treisman, 2009; Dux et al. 2010).
Object substitution is thought to be based solely on re-entrant processing (Enns & Di Lollo, 2000; Enns, 2004), in which the representation of the target is replaced with the representation of the mask. Hence, if patients with schizophrenia show abnormalities in a paradigm that is limited to masking by object substitution, it would implicate aberrant re-entrant processes. However, all of the masking paradigms used in schizophrenia research have involved both object formation and object substitution components. There is one specialized procedure, four-dot masking, which involves only masking by object substitution (Enns, 2004), but it has not been previously applied to schizophrenia. In this paradigm, the mask consists of four relatively small dots, one at each corner of the target. This type of mask provides no contour information and does not overlap with the target. Hence, it cannot integrate with the target in a way that would disrupt identification.
In this project, we administered three visual processing procedures to characterize better visual perceptual abnormalities in schizophrenia. These procedures included: (1) forward and backward location masking with overlapping target and mask stimuli; (2) four-dot masking; (3) a cuing procedure with a single dot that does not mask the target. The cuing procedure with a single dot was included to assess iconic decay. Four-dot masking involves longer processing delays than location masking and depends on maintaining an iconic representation (i.e. visible persistent) of the target. Hence, if patients and controls differ in their performance on four-dot masking, it could possibly be explained by different rates of iconic decay. Four-dot masking can have a delayed onset of the mask, or it can have the same onset as the target and a delayed offset (called common-onset masking). For the purposes of this study, we used the delayed onset version so that it would be more comparable with the location masking.
Using these three procedures we addressed the following goals. First, we wanted to see whether we could replicate our previous findings that schizophrenia patients show impairment on location masking after they have been equated for sensory input of the target. Second, we used a four-dot masking procedure to evaluate whether the groups differed in masking by object substitution. Third, we used a cuing task to determine whether the patients and controls differed in their rates of visible persistence/iconic decay. We predicted that patients would show increased susceptibility to the mask in four-dot masking performance but normal rates of visible persistence.
Method
Participants
A total of 136 (29 female) patients were recruited from out-patient treatment clinics at the Veterans Affairs Greater Los Angeles Healthcare System and through presentations at residences in the community. Patients met criteria for schizophrenia (n=124) or schizoaffective disorder (n=12) based on the SCID Axis I Disorders (First et al. 1997). Altogether, 112 patients were receiving atypical antipsychotic medications, seven were receiving typical antipsychotic medications, nine were receiving both types of medication and eight were not taking an antipsychotic medication at time of assessment.
In total, 79 (20 female) healthy control participants were recruited through newspaper and Internet advertisements. Control participants were screened with the SCID and SCID-II (First et al. 1996) and were excluded if they met criteria for any lifetime psychotic disorder, bipolar mood disorder, recurrent depression, substance dependence, paranoid, schizotypal or schizoid personality disorder and any evidence (according to participant report) of a history of psychotic disorder among their first-degree relatives. Additional exclusion criteria for both groups included aged <18 or >60 years, active substance use disorder in the past 6 months, identifiable neurological disorder, IQ <70, history of loss of consciousness for>1 h or insufficient fluency in English. All participants had the capacity to give informed consent and provided written informed consent after all procedures were fully explained in accordance with procedures approved by the Institutional Review Boards at UCLA and the Veterans Affairs Greater Los Angeles Healthcare System.
Clinical symptom ratings were conducted with the expanded 24-item UCLA version of the Brief Psychiatric Rating Scale (Ventura et al. 1993b) and with the Scale for the Assessment of Negative Symptoms (Andreasen, 1984). All clinical interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center based on established procedures (Ventura et al. 1993a, 1998). The process included formal didactics, achieving a minimum level of reliability using an extensive library of videotaped interviews, as well as live, co-rated interviews conducted with faculty members. After certification, all raters participated in a continuous quality assurance programme that involved periodic reliability checks and co-rated live interviews with faculty.
Procedures
Participants performed three different visual processing tasks. The first task was identifying the location of a target (location masking) and consisted of both forward and backward masking conditions. The two other tasks (four-dot masking and cuing task) used only a backward masking format. All stimuli were presented using E-Prime 1.1 software (Psychological Software Tools, USA) on a 17 inch cathode ray tube computer monitor running at a resolution of 640 × 480 pixels and a 160 Hz refresh rate, yielding a screen sweep of 6.25 ms. Participants sat 1 m away from the monitor. Procedures were administered in a set order: location masking; four-dot masking; the cuing task.
Location masking
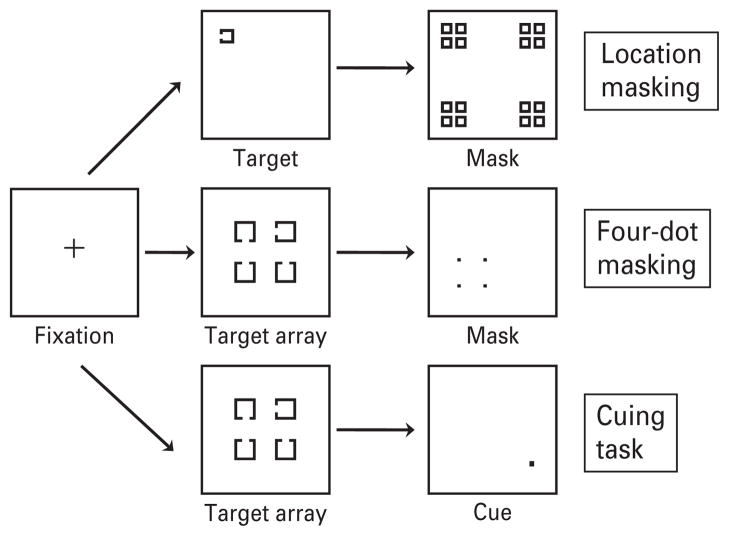
The location masking task was based on previous studies reported in our laboratory and reported in detail elsewhere (Green et al. 2002, 2003b). The target consisted of a single square with a notch that could appear at the top, bottom or left side of the square. The target could appear at one of four different locations, arranged in a notional square, on the computer screen. The masking stimulus consisted of a pattern of squares that occupied every possible target location. Targets measured 0.27 × 0.27° of visual angle. The mask measured 2.01 × 2.01° of visual angle. Examples of the stimuli are shown in Fig. 1.
Fig. 1.
The stimuli are shown for each of the visual processing paradigms. Location masking is on top, four-dot masking in the middle and cuing on the bottom.
The target was presented for 12.5 ms (two screen sweeps) and the mask was presented for 25 ms (four screen sweeps). As in previous masking studies, we first used a psychophysical procedure [critical stimulus intensity (CSI)] to equate the participants on the target threshold (Green et al. 2002). This procedure moves the contrast level up and down according to the subject’s performance so that each subject is performing at 84% accuracy for identifying the unmasked target. The gray scale value determined in this CSI procedure is then used for all of the stimuli in the location masking task. In location masking, 12 trials were presented for each stimulus onset asynchrony (SOA; the interval between the onset of the target stimulus and the onset of the mask). Both forward and backward masking was assessed. In forward masking the mask preceded the target, whereas in backward masking the mask followed the target. Six SOAs (12.5, 25.0, 37.5, 50.0, 62.5, 75 ms) were used in both the forward and backward masking tasks. Additionally, an SOA of 0 ms was used (i.e. simultaneous target and mask onset). This masking condition typically yields a monotonic masking function, in which performance is lowest at a SOA of 0 ms.
Each trial started with a fixation cross presented for 400 ms. A blank screen was then presented for 500 ms. Following the blank screen, a forward or backward masking trial was presented. Participants verbally reported in which one of the four quadrants they believed the target appeared and the experimenter entered their response. The experimenter then initiated the next trial. A schematic of a trial can be seen in Fig. 1.
Four-dot masking
The four-dot masking procedure was modified from similar tasks described elsewhere (Enns & Di Lollo, 2000; Enns, 2004). In this masking condition, four potential targets appeared in a notional square on the computer monitor followed by a mask surrounding one of the potential targets (see Fig. 1). The mask essentially cues which target the participant was supposed to identify. The target array consisted of four squares with a notch missing from either the top, bottom or left side of the square. The mask consisted of four dots that surround, but do not touch, one of the potential targets. Each potential target measured 1.55 × 1.55° of visual angle and was arranged in a square of 4.58 × 4.58° of visual angle. The four-dot mask measured 2.23 × 2.23° of visual angle and each dot in the mask subtended 0.23 × 0.23° of visual angle. The target was presented for 25 ms and the mask was presented for 37.5 ms. The target stimuli were black, presented on a white background and were supra-threshold for all subjects, unlike the location masking condition that was set to an individual’s threshold. In total, 12 trials were presented for each of eight SOAs (0, 25, 50, 75, 100, 125, 150, 175 ms). In four-dot masking, performance typically decreases with increasing SOAs, unlike the pattern for location masking.
A trial started with a fixation cross presented for 450 ms, followed by a blank screen presented for 500 ms. Following the blank screen, the target and masking stimuli, separated by the above-mentioned SOAs, were presented. Participants verbally reported the direction of the notch (up, side or down) of the target that was surrounded by the four-dot mask and the experimenter entered their response. The next trial was then initiated by the experimenter.
Cuing task
A cuing task was used to assess rate of iconic decay (visible persistence). The cuing procedure used the exact same target stimuli and SOAs as the four-dot masking procedure except that instead of four dots only a single dot was used, which does not act as a mask. The single dot measured 0.34 × 0.34° of visual angle. Examples of the stimuli are shown in Fig. 1.
Data analysis
Accuracy was defined as percent correct (out of 12 trials) at each separate SOA for each visual processing procedure. For location masking, a two (group) × two (forward/backward) analysis of variance (ANOVA) was run. The value for forward masking was the mean of the six forward SOAs and the value for backward masking was the mean of the six backward SOAs; SOA=0 ms was not included. Because the masking function for location task in patients is well-characterized, we considered only the mean forward and mean backward values. In contrast, the function for four-dot masking in schizophrenia was unknown, so we conducted a two (group) × eight (SOA) ANOVA separately for the four-dot and cuing procedures. All statistical analyses used an a priori two-tailed significance level of 0.05 to determine significant results.
Results
Demographic information can be seen in Table 1. Patients were clinically stable with relatively low levels of symptoms. They were older than controls and had somewhat less parental education. Visual masking is known to be associated with age (Green et al. 2003b) and preliminary analyses confirmed that age was associated with performance on the paradigms in this study. The effect of age on performance was not different between the two groups. Hence, we used age as a covariate in the subsequent analyses. There were no significant associations between parental education and performance on any task.
Table 1.
Demographic information
| Schizophrenia patients (n=136)
|
Normal controls (n=79)
|
|||
|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |
| Agea | 46.3 | 9.9 | 39.0 | 9.7 |
| Parental education | 12.6 | 3.0 | 13.4 | 2.3 |
| Percent female | 21 | 25 | ||
| BPRS | ||||
| Total score | 44.3 | 8.9 | ||
| Factors (mean score per item) | ||||
| Depression/Anxiety | 2.0 | 0.8 | ||
| Thinking disturbance | 2.8 | 1.3 | ||
| SANS Global Scores | ||||
| Affective flattening | 1.6 | 1.3 | ||
| Alogia | 0.7 | 1.1 | ||
| Avolition | 2.2 | 1.4 | ||
| Anhedonia | 2.7 | 1.7 | ||
BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms.
p<0.01 for difference between controls and patients.
Location masking
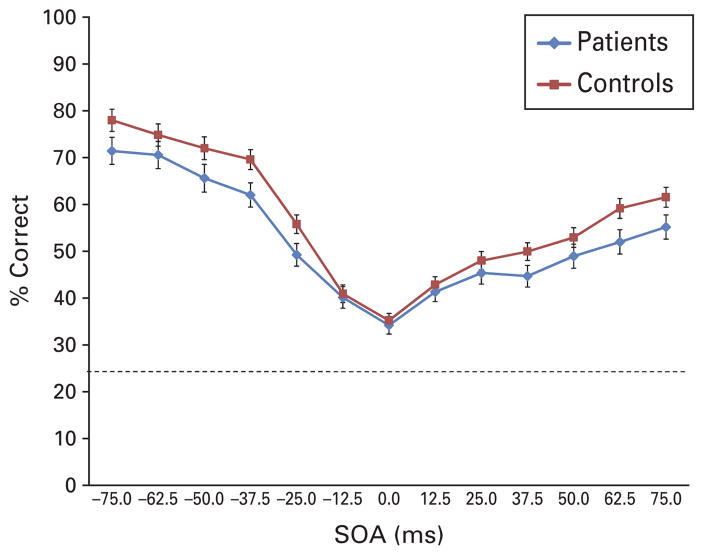
Out of the original sample, 16 patients and five controls did not perform the CSI procedure in a way that reliably converged on a threshold and these subjects were not given the location masking task. The schizophrenia sample required more contrast (higher gray scale value) than controls to achieve comparable visual input thresholds; mean (S.D.) value was 33.8 (15.3) for patients and 26.1 (14.8) for controls [t(192)=3.48, p<0.01]. For location masking, the two (group) × two (forward versus backward masking) analysis of covariance (ANCOVA) revealed a significant main effect of group [F(1, 191)=6.64, p<0.05]. This result replicates previous findings of impairment on this and similar masking procedures. The main effect of forward/backward and the interaction was not significant. The means for each SOA and standard errors, adjusted for age, are shown in Fig. 2.
Fig. 2.
The performance of patients and healthy controls on location masking after being matched for identification of unmasked targets. Values have been adjusted for age. On the x-axis, the negative stimulus onset asynchrony (SOA) values indicate forward masking and the positive values indicate backward masking. The figure includes bars for standard errors at each SOA and the dotted line shows chance performance at 25%.
Four-dot masking and cuing task
Before analyzing data from these tasks, we dropped subjects who were considered to have invalid performance. Specifically, we dropped subjects if they got <18 trials correct over the first three SOAs for the cuing task (i.e. <50% correct of the first three SOAs). This criterion was selected because: (1) the cuing task is the easier of the two tasks; (2) both of these tasks become harder at longer SOAs (unlike the location task) and performance reached asymptote after the first three SOAs; (3) the probability of getting ≥18 trials correct solely by chance is<0.05 based on a binomial distribution. Altogether, 12 patients and two controls were excluded from analyses using this criterion. In addition, data were missing for three patients on the four-dot masking procedure.
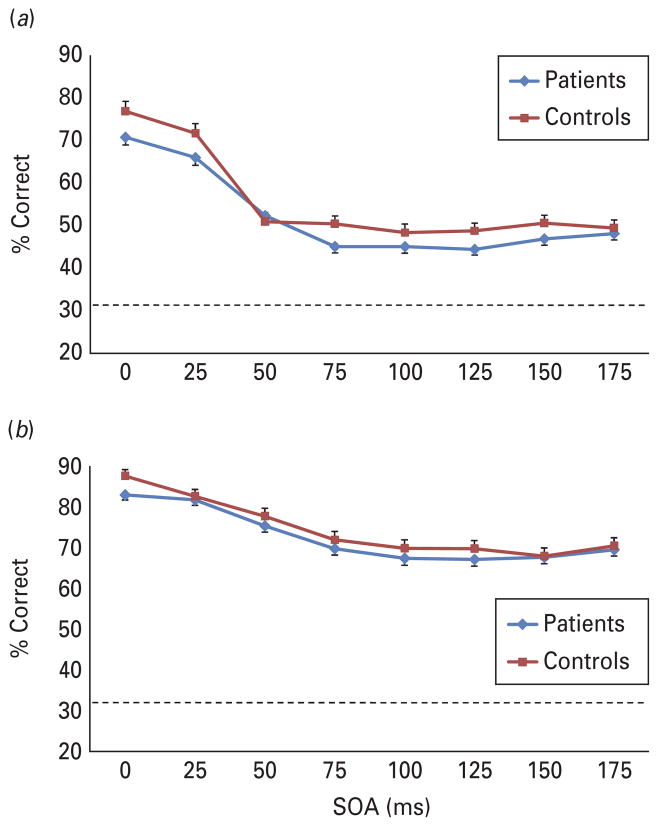
For the four-dot masking, both groups showed the expected decrease in performance with increasing SOAs (see Fig. 3a). The two (group) × eight (SOA) ANCOVA revealed a significant main effect of SOA [F(7, 1365)=7.84, p<0.001]. There was also a significant main effect of group [F(1, 195)=4.05, p<0.05]. The interaction between group and SOA was not significant. These results reveal that schizophrenia patients were more susceptible to masking by object substitution compared with healthy controls.
Fig. 3.
(a) The performance of each group, adjusted for age, on (a) the four-dot masking procedure and (b) the cuing task. Mask (for four-dot) or cue (for cuing) were always shown after the target (backward masking format). The groups differed significantly on four-dot masking, but not the cuing task. The figure includes bars for standard errors at each stimulus onset asynchrony (SOA) and the dotted line shows chance performance at 33%.
We next evaluated the cuing procedure to determine whether the groups differed in their rate of iconic decay. The two (group) × eight (SOA) ANCOVA revealed no significant main effects or interactions. Although the overall ANCOVA for the SOA effect was not significant, the linear contrast for the target decay over time was significant [F(1, 198)=8.00, p<0.01]. The linear contrast by group interaction was not significant. These results (Fig. 3b) indicate that both groups showed comparable rates of decay.
Discussion
This paper yielded several key findings. First, we replicated previous findings of visual masking impairment in schizophrenia with a task that is believed to interrupt object formation. This impairment occurred after subjects were matched for sensory input of unmasked targets using a threshold procedure. Second, the groups did not differ in their iconic decay rates on a cuing task. Third, the patients differed from controls on four-dot masking, which is believed to work only through masking by object substitution. Masking by object substitution, thought to depend on re-entrant activation, occurs at higher levels of processing than other forms of masking. For example, object substitution masking can interfere at levels as high as those required for semantic processing (Reiss & Hoffman, 2006). Moreover, a recent study showed that object substitution masking occurs at levels higher than backward masking by (spatially overlapping) noise or by meta-contrast (Chakravarthi & Cavanagh, 2009).
We had predicted that patients and controls would be comparable in iconic decay. The most common way to assess iconic decay in the cognitive science literature is with a partial report procedure, in which a participant is cued about which part of a stimulus array to report. The cuing procedure in this study can be considered a modified partial report task. The partial report procedure has only been used twice before in schizophrenia research; in an early study (Knight et al. 1977) and in a recent paper (Hahn et al. 2010). The results of the paper from Knight et al. are complicated by the inclusion of three subgroups of schizophrenia patients and the absence of a healthy control group, but it appears that iconic decay was normal in at least one subgroup of patients. The results from Hahn et al. showed comparable iconic decay rates in patients and healthy controls, consistent with the current study. Our finding of normal iconic decay in schizophrenia patients implies that any group differences in masking cannot be accounted for by different decay rates. Because a normal rate of iconic decay was observed on the paradigm that was always administered last, differential fatigue cannot explain the pattern of results. Differential improvement in patients over the testing session, though unlikely, is a possibility.
Given the absence of differences in iconic decay, we can speculate that the observed impairment in fourdot masking might be due to other possible mechanisms. One possibility is that the well-replicated finding of problems in masking by disruption of object formation carries forward into the later-stage masking by object substitution. In this case, a single initial abnormality in constructing a visual percept would provide a weakened visual representation for the next stage of processing. An alternative explanation is that a separate abnormality affects re-entrant processes and creates a situation in which patients are more susceptible to masking by object substitution. This second explanation would suggest two separate visual processing abnormalities. It also suggests that the differences between groups would become larger as the four-dot masking delay increases (that is, larger group differences over longer SOAs). The lack of a group by SOA interaction fails to support this prediction.
Neuroimaging studies have provided insights into the neural basis for object formation and recognition, which, in turn, suggest a possible basis for abnormalities in visual processing in schizophrenia. Studies with healthy controls have demonstrated that area LO is closely tied to perceiving a masked target (Grill- Spector et al. 2000; Bar et al. 2001; Green et al. 2005; Carlson et al. 2007). Hence, area LO appears to be integral for object recognition. Using functional magnetic resonance imaging, we found that patients have a blunted area LO response to masked stimuli across SOAs, suggesting that a lower, or less organized, response in area LO may be a neural basis for impairments in a variety of visual processing tasks that require intact object formation (Green et al. 2009).
The results from this study can be applied more generally to models of outcome in schizophrenia. Most studies that attempt to model pathways to outcome in schizophrenia have not included perceptual measures. However, studies from our laboratory and others have found that perceptual processing measures fit well into these models and are consistent with bottom-up theoretical formulations (Leitman et al. 2005, 2010; Sergi et al. 2006; Javitt, 2009; Rassovsky et al. 2010). For example, we have found that visual processing in schizophrenia is associated with social cognition, which is associated, in turn, with community functioning (Sergi et al. 2006; Rassovsky et al. 2010). Similarly, other studies of early auditory perception have reported linkages to auditory measures of social cognition (Leitman et al. 2005). It remains to be seen whether the information provided from object substitution masking helps to account for variance in these models that is not attributed to masking associated with object formation.
In summary, it appears that visual processing in schizophrenia is characterized by an impairment that includes not only object formation processes, but also later-stage masking by object substitution. While the results from this paper indicate the presence of an impairment in masking by object substitution, this study cannot determine whether this impairment constitutes a new and separate impairment (perhaps involving re-entrant processes) or a continuation of the known deficit in object formation. Ongoing studies with neuroimaging and electrophysiology will help to make this determination.
Acknowledgments
Support for this study came from NIMH Grants MH043292 and MH065707 (PI: Michael F. Green, Ph.D.).
Footnotes
Declaration of Interest
None.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Bar M, Tootell RB, Schacter DL, Greve DN, Fischl B, Mendola JD, Rosen BR, Dale AM. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–535. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Bedwell JS, Brown JM, Miller S. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophrenia Research. 2003;63:273–284. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG. Visual Masking: An Integrative Approach. Oxford University Press; New York: 1984. [Google Scholar]
- Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: a comparison, review, and update. Perception and Psychophysics. 2000;62:1572–1595. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the VBM deficits of schizophrenia patients. Biological Psychiatry. 1998;43:132–138. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Carlson TA, Rauschenberger R, Verstraten FA. No representation without awareness in the lateral occipital cortex. Psychological Science. 2007;18:298–302. doi: 10.1111/j.1467-9280.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- Chakravarthi R, Cavanagh P. Recovery of a crowded object by masking the flankers: determining the locus of feature integration. Journal of Vision. 2009;9:1–9. doi: 10.1167/9.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Treisman A. Implicit perception and level of processing in object-substitution masking. Psychological Science. 2009;20:560–567. doi: 10.1111/j.1467-9280.2009.02328.x. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Enns JT, Rensink RA. Competition for consciousness among visual events: the psychophysics of reentrant visual processes. Journal of Experimental Psychology: General. 2000;129:481–507. doi: 10.1037//0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- Dux PE, Visser TAW, Goodhew SC, Lipp OV. Delayed reentrant processing impairs visual awareness: an object-substitution-masking study. Psychological Science. 2010;21:1242–1247. doi: 10.1177/0956797610379866. [DOI] [PubMed] [Google Scholar]
- Enns JT. Object substitution and its relation to other forms of visual masking. Vision Research. 2004;44:1321–1331. doi: 10.1016/j.visres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Enns JT, Di Lollo V. What’s new in visual masking? Trends in Cognitive Science. 2000;4:345–352. doi: 10.1016/s1364-6613(00)01520-5. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1997. [Google Scholar]
- Green MF, Glahn D, Engel SA, Nuechterlein KH, Sabb F, Strojwas M, Cohen MS. Regional brain activity associated with visual backward masking. Journal of Cognitive Neuroscience. 2005;17:13–23. doi: 10.1162/0898929052880011. [DOI] [PubMed] [Google Scholar]
- Green MF, Lee J, Cohen MS, Engel SA, Korb AS, Nuechterlein KH, Wynn JK, Glahn DC. Functional neuroanatomy of visual masking deficits in schizophrenia. Archives of General Psychiatry. 2009;66:1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Mintz J, Salveson D, Nuechterlein KH, Breitmeyer BG, Light GA, Braff DL. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biological Psychiatry. 2003a;53:1113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenia patients: evidence for a vulnerability indicator. Archives of General Psychiatry. 1997;54:465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B. Development of a computerized assessment for visual masking. International Journal of Methods in Psychiatric Research. 2002;11:83–89. doi: 10.1002/mpr.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflections of aberrant cortical oscillations. American Journal of Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biological Psychiatry. 2006;59:446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Tsuang J, Mintz J. Forward and backward visual masking in schizophrenia: influence of age. Psychological Medicine. 2003b;33:887–895. doi: 10.1017/s003329170200716x. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: specifying a mechanism. Archives of General Psychiatry. 1994a;51:939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania: specifying the visual channels. Archives of General Psychiatry. 1994b;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-selective activation correlate with recognition performance in humans. Nature Neuroscience. 2000;3:837–843. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- Hahn B, Kappenman ES, Robinson BM, Fuller RL, Luck SJ, Gold JM. Iconic decay in schizophrenia. Schizophrenia Bulletin. 2010 doi: 10.1093/schbul/sbp164. Published online: 6 January 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annual Review of Clinical Psychololgy. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychological Medicine. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Knight R, Sherer M, Shapiro J. Iconic imagery in overinclusive and non-overinclusive schizophrenics. Journal of Abnormal Psycholology. 1977;86:242–255. doi: 10.1037//0021-843x.86.3.242. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biological Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. American Journal of Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- Lieb K, Denz E, Hess R, Schuttler R, Kornhuber HH, Schreiber H. Preattentive information processing as measured by backward masking and texton detection tasks in adolescents at high genetic risk for schizophrenia. Schizophrenia Research. 1996;21:171–182. doi: 10.1016/0920-9964(96)00025-4. [DOI] [PubMed] [Google Scholar]
- Miller S, Saccuzzo D, Braff D. Information processing deficits in remitted schizophrenics. Journal of Abnormal Psychology. 1979;88:446–449. [PubMed] [Google Scholar]
- Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychological Medicine. 2010;19:1–11. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss JE, Hoffman JE. Object substitution masking interferes with semantic processing: evidence from event-related potentials. Psychological Science. 2006;17:1015–1020. doi: 10.1111/j.1467-9280.2006.01820.x. [DOI] [PubMed] [Google Scholar]
- Ro T, Breitmeyer B, Burton P, Singhal NS, Lane D. Feedback contributions to visual awareness in human occipital cortex. Current Biology. 2003;13:1038–1041. doi: 10.1016/s0960-9822(03)00337-3. [DOI] [PubMed] [Google Scholar]
- Rund BR. Backward-masking performance in chronic and nonchronic schizophrenics, affectively disturbed patients, and normal control subjects. Journal of Abnormal Psychology. 1993;102:74–81. doi: 10.1037//0021-843x.102.1.74. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia: new findings using schizophrenic subgroups and manic control subjects. Archives of General Psychiatry. 1981;38:175–179. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, Braff DL. Information-processing abnormalities. Schizophrenia Bulletin. 1986;12:447–459. doi: 10.1093/schbul/12.3.447. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, Schubert DL. Backward masking as a measure of slow processing in schizophrenia spectrum disorders. Journal of Abnormal Psychology. 1981;90:305–312. doi: 10.1037//0021-843x.90.4.305. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo GVZ, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophrenia Research. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schuck JR, Lee RG. Backward masking, information processing, and schizophrenia. Schizophrenia Bulletin. 1989;15:491–500. doi: 10.1093/schbul/15.3.491. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. American Journal of Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: ‘The Drift Busters’. International Journal of Methods in Psychiatric Research. 1993a;3:221–224. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A. Training and quality assurance with the Structured Clinical Interview for DSM-IV. Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993b;3:227–243. [Google Scholar]
- Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward masking task. American Journal of Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]