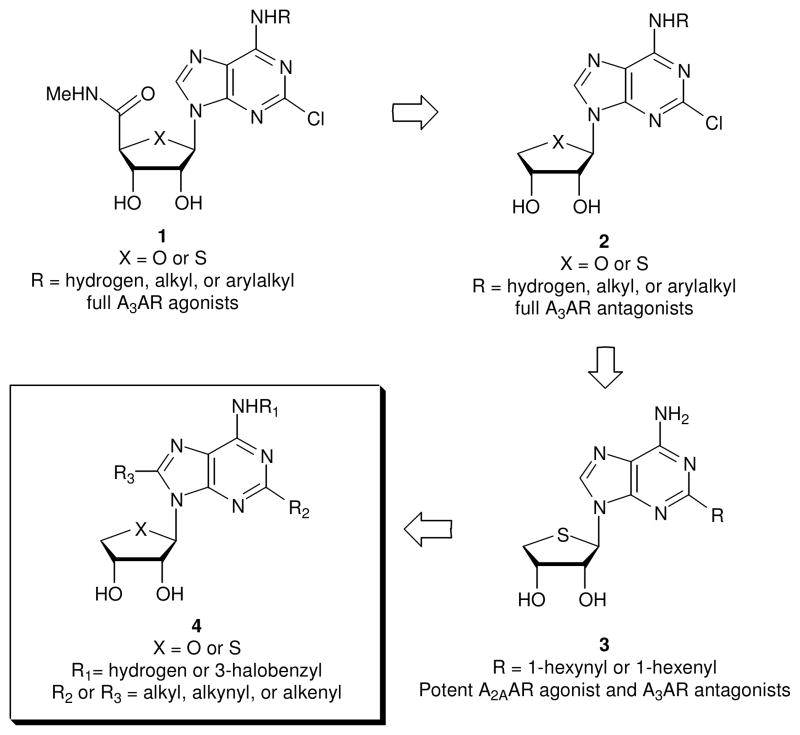
Figure 1.
The design of the target nucleosides acting dually at the A2A and A3ARs A molecular modeling study8 indicated that the NH of the 5′-uronamide of 1 serves as a hydrogen bonding donor in the A3AR binding site and is associated with the induced fit for the receptor activation. On this basis, we designed and synthesized truncated adenosine derivatives 29 removing the 5′-uronamide of 1, to bind potently but with altered ability to induce the conformational change essential for receptor activation. As expected, members of the series of compound 2 proved to be potent and selective A3AR antagonists.9 It should be noted that these A3AR antagonists 2 also showed species-independent binding affinity, making them suitable for efficacy evaluation for drug development in small animal models.9 Compound 2 (X = S, R = 3-iodobenzyl) exhibited a potent anti-glaucoma effect in vivo.10