Table 1.
Binding affinities of known A3AR antagonist 2 and truncated C2- and C8-substituted derivatives 4a–4r at three subtypes of hARs and A3AR-mediated inhibition of cAMP production
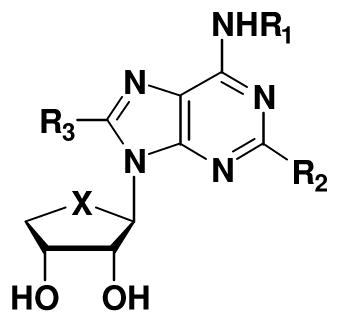 | ||||
|---|---|---|---|---|
| Compounds (R1, R2, R3) | Affinity (Ki, nM ± SEM, or % inhibition)a | Relative efficacy (%inhibition of cAMP ± SEM)c | ||
| hA1 | hA2A | hA3 | hA3 | |
| 2 (X = S, R1 = 3-I-Bn, R2 = Cl, R3 = H)b | 2490 ± 940 | 341 ± 75 | 4.16 ± 0.50 | 4.2 ± 2.3 |
| 4a (X = O, R1 = H, R2 = 2-hexynyl, R3 = H) | 740 ± 430 | 63.2 ± 15 | 138 ± 44 | 17.4 ± 2.4 |
| 4b (X = O, R1 = 3-F-Bn, R2 = 2-hexynyl, R3 = H) | 9 ± 1% | 25 ± 3% | 570 ± 130 | 29.5 ± 5.5 |
| 4c (X = O, R1 = 3-Cl-Bn, R2 = 2-hexynyl, R3 = H) | 9 ± 5% | 42 ± 13% | 150 ± 140 | 39.7 ± 3.8 |
| 4d (X = O, R1 = 3-Br-Bn, R2 = 2-hexynyl, R3 = H) | 12 ± 4% | 27 ± 4% | 67 ± 18 | 37.2 ± 2.9 |
| 4e (X = O, R1 = 3-I-Bn, R2 = 2-hexynyl, R3 = H) | 18 ± 2% | 32 ± 9% | 220 ± 50 | 31.5 ± 2.8 |
| 4f (X = O, R1 = H, R2 = 2-hexanyl, R3 = H) | 10 ± 5% | 2160 ± 270 | 39 ± 5% | 22.3 ± 4.5 |
| 4g (X = S, R1 = H, R2 = 2-hexynyl, R3 = H) | 39 ± 10% | 7.19 ± 0.6 | 11.8 ± 1.3 | 2.8 ± 1.6 |
| 4h (X = S, R1 = 3-F-Bn, R2 = 2-hexynyl, R3 = H) | 24 ± 5% | 46 ± 5% | 150 ± 80 | 47.6 ± 4.9 |
| 4i (X = S, R1 = 3-Cl-Bn, R2 = 2-hexynyl, R3 = H) | 25 ± 2% | 3730 ± 200 | 24.0 ± 6.0 | 47.2 ± 3.4 |
| 4j (X = S, R1 = 3-Br-Bn, R2 = 2-hexynyl, R3 = H) | 12 ± 1% | 3910 ± 970 | 24.0 ± 5.0 | 25.2 ± 3.0 |
| 4k (X = S, R1 = 3-I-Bn, R2 = 2-hexynyl, R3 = H) | 15 ± 3% | 4890 ± 840 | 39 ± 5 | 40.0 ± 5.6 |
| 4l (X = O, R1 = H, R2 = 2-hexenyl, R3 = H) | 31.9 ± 1.2% | 178 ± 26 | 218 ± 79 | 42.6 ± 3.1 |
| 4m (X = S, R1 = H, R2 = 2-hexenyl, R3 = H) | 16.2 ± 8.4% | 72.0 ± 19.1 | 13.2 ± 0.8 | 10.7 ± 4.1 |
| 4n (X = O, R1 = H, R2 = H, R3 = 2-hexynyl) | 290 ± 70 | 27.2 ± 2.9% | 31.7 ± 7.4 | 24.5 ± 2.9 |
| 4o (X = S, R1 = H, R2 = H, R3 = 2-hexynyl) | 49.3 ± 4.9% | 46.5 ± 4.3% | 20.0 ± 4.0 | 1.7 ± 3.9 |
| 4p (X = O, R1 = H, R2 = H, R3 = 2-hexanyl) | 18.1 ± 5.0% | 5.8 ± 4.6% | 24.7 ±2.1% | 12.9 ± 2.2 |
| 4q (X = O, R1 = H, R2 = H, R3 = 2-hexenyl) | 500 ± 140 | 27.3 ± 6.3% | 94.2 ± 30.0 | 19.5 ± 2.4 |
| 4r (X = S, R1 = H, R2 = H, R3 = 2-hexenyl) | 3.7 ± 2.9% | 22.8 ± 6.4% | 259 ± 10 | 6.1 ± 1.7 |
All binding experiments were performed using adherent mammalian cells stably transfected with cDNA encoding the appropriate hAR (A1AR and A3AR in CHO cells and A2AAR in HEK-293 cells). Binding was carried out using 1 nM [3H]-R-(-)-N6-2- phenylisopropyl adenosine (R-PIA), [3H]-2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamidoadenosine (26, CGS21680, 10 nM), or 0.5 nM [125I]-N6-(3-iodo-4-aminobenzyl)-5′-N-methylcarboxamidoadenosine (27, I-AB-MECA) as radioligands for A1, A2A, and A3ARs, respectively. Values are expressed as mean ± sem, n = 3–4 (outliers eliminated) and normalized against a non-specific binder 25 (10 μM). Values expressed as a percentage in italics refer to percent inhibition of specific radioligand binding at 10 μM, with nonspecific binding defined using 10 μM 25.
Ref. 1.
Maximal efficacy (at 10 μM) in an A3AR functional assay, determined by inhibition of foskolin-stimulated cAMP production in AR-transfected CHO cells, expressed as percent inhibition (mean ± standard error, n = 3 – 5) in comparison to effect (100%) of full agonist 1a at 10 μM.
Data for compounds 4g, 4m, 4o, and 4r are from reference 11
