Figure 4. Shared Binding Mode of Duf1 and Cdc4 β-Propellers to Ub.
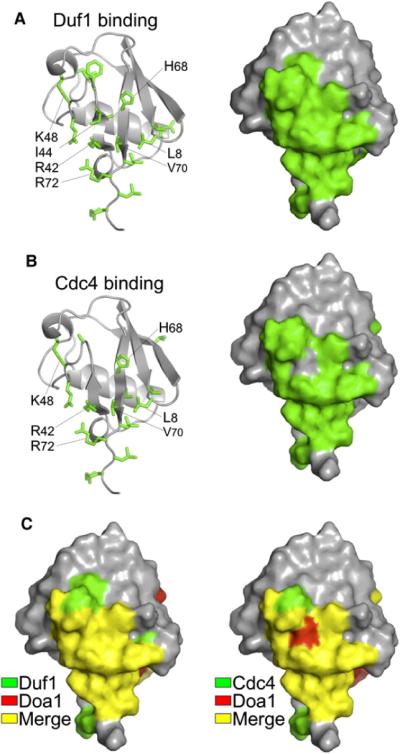
(A) Mapping of chemical shift perturbation data onto Ub from HSQC data of 15N-labeled Ub in the presence and absence of unlabelled β-Prp from Duf1 (1:15). Left shows side chains of affected residues, and right shows corresponding molecular surface.
(B) Similar analysis as in (A) for Cdc4:Skp1 complex at a 6:1 ratio with Ub.
(C) The Ub binding surfaces, as defined by NMR chemical shift perturbation, used by Duf1 (left) and Cdc4 (right) compared with that used by Doa1 β-Prps. Residues involved in Duf1 and Cdc4 binding are in green, those involved in Doa1 binding are in red, and those in common (merge) are in yellow.
