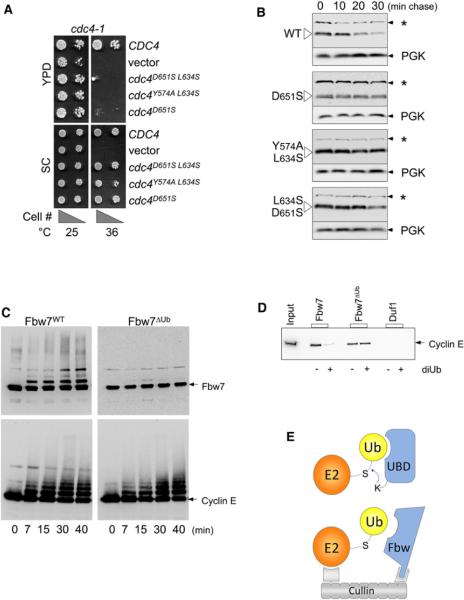
Figure 6. Ub Binding Promotes Ubiquitination and Degradation of F Box Proteins.
(A) Temperature-sensitive cdc4-1 cells were transformed with low-copy-expressing wild-type HA-epitope-tagged Cdc4 (CDC4), no Cdc4 (vector), or Cdc4ΔUb mutants with the indicated residue changes that block Ub binding by the β-Prp. Expression was driven by the CDC4 promoter. Cell dilutions were plated onto SC minimal media plates or YPD-rich media plates and grown at 25°C or 36°C, which inactivates the endogenous Cdc4-1 protein.
(B) Cells expressing wild-type HA-Cdc4 and the indicated HA-Cdc4ΔUb mutant cells were subjected cycloheximide chase experiments to estimate protein half-life. At the indicated times after cycloheximide addition, aliquots were processed and analyzed by immunoblotting with anti-HA antibodies. As internal loading controls, blots were subsequently immunoblotted for Pgk1. Our anti-HA blotting conditions also revealed a constant background band (*) that provided an additional loading standard.
(C) In vitro ubiquitination assays with WT Skp1:Fbw7 and mutant Fbw7ΔUbD560S,D600S. Skp1:Fbw7 complexes (1 μM) were incubated with 50 μM Ub, 0.2 μM E1, 5 mM ATP, 2 μM each UbcH5 and hCdc34, 1 μM Rbx1:Cul1Nedd8 complex, and 0.5 μM cyclin-E/CDK2 at 30°C. Aliquots were taken at the indicated time points, diluted with SDS-containing sample buffer, and immunoblotted for cyclin-E and Fbw7 using anti-cyclin E and anti-Fbw7 antibodies.
(D) WT Skp1:Fbw7, mutant Skp1:Fbw7ΔUbY545A, L583S, or the Duf1 β-Prp was covalently attached to Sepharose beads and incubated with cyclin-E/CDK complex in PBS containing 5 mM MgCl2, 1 mM ATP, 10 nM cyclin-E/CDK, and 0.05 mg/ml BSA in a volume of 200 μl in the presence or absence of 120 μM diUb.
(E) Model for how Ub binding could promote ubiquitination and degradation of F box proteins. Above shows a model (based on Hoeller et al., 2007; Sorkin, 2007) for E3-independent ubiquitination of proteins containing a ubiquitin-binding domain (UBD). The UBD would bind Ub~E2, facilitating attack from an acceptor lysine residue within the UBD-containing protein. Below is the same scheme, except that this would happen within the context of a fully assembled SCF complex. The close proximity of the E2 to the F box protein afforded by the Cullin/Skp1/Rbx1 complex would facilitate E2~Ub:β-Prp interaction and compensate for the modest affinity that F box β-Prps show independently. Binding of substrate might occlude Ub interaction, thereby prolonging the life of the F box protein until available substrate is depleted.