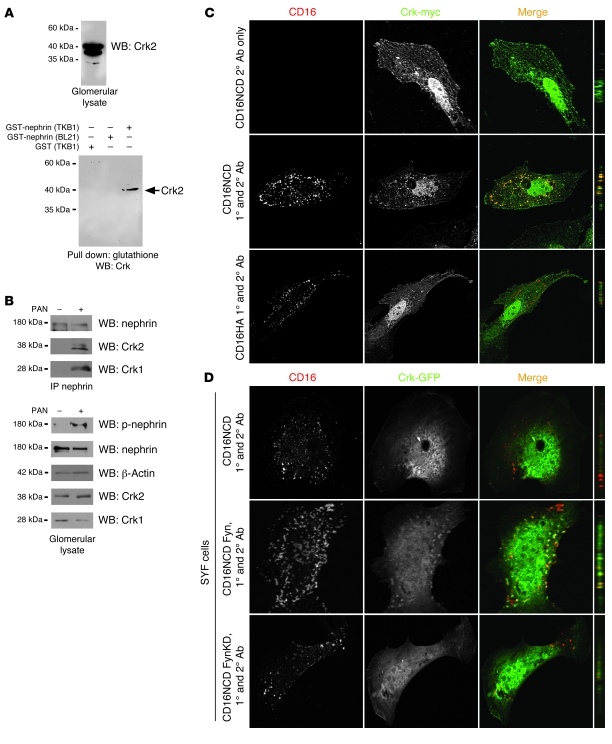
Figure 1. Crk1/2 interacts with nephrin in a tyrosine phosphorylation–dependent fashion.
(A) Purified recombinant GST-nephrinCD expressed in BL21 or TKB1 E. coli (to produce nonphosphorylated or tyrosine-phosphorylated nephrin, respectively) or purified GST alone was mixed with rat glomerular lysate, pulled down with glutathione agarose, and immunoblotted with monoclonal anti-Crk1/2 antibody. (B) Glomerular lysates from rats injected with PBS (control) or PAN were immunoprecipitated and/or immunoblotted using the indicated antibodies. (C) Cultured human podocytes expressing CD16/7-nephrinCD (CD16NCD) or CD16/7-HA (CD16HA) and Crk2-myc were activated by clustering: namely, addition of monoclonal anti-CD16 primary antibody (1°) and/or goat anti-mouse IgG Texas Red–conjugated secondary antibody (2°), as indicated, to the media of live cells. Crk2-myc was detected with rabbit polyclonal anti-myc primary antibody and Alexa Fluor 488–labeled secondary antibody. Cells were analyzed by confocal microscopy. CD16/7-nephrinCD (red) and Crk2-myc (green) colocalized in the plane of the plasma membrane. (D) SYF MEFs transiently expressing CD16/7-nephrinCD and Crk-GFP were activated by clustering. Although colocalization of nephrin and Crk was not observed in SYF MEFs, nephrin-Crk association was rescued by reexpressing Fyn, but not by expressing a kinase-dead variant of Fyn (FynKD). (C and D) Original magnification, ×630. yz plane reconstructions are shown at far right.