Abstract
The mechanisms underlying gender differences in stroke incidence, risk, and outcome are uncertain. We sought to determine whether transcriptional profiles of circulating blood cells of men and women differentially correlated with carotid artery intima–media thickness (CIMT), a predictor of atherosclerosis and stroke risk. Gene expression in whole blood was measured using Affymetrix expression arrays in men (n=17) and women (n=35), aged 45–64 years, with at least one risk factor for stroke. Mean average CIMT was measured using B-mode ultrasound. Expression levels of 746 genes positively and 292 genes negatively correlated with CIMT only in women (p<0.05); 881 genes positively and 597 genes negatively correlated with CIMT only in men (p<0.05). Forty-one genes correlated with CIMT in men and women, but in opposite directions. These genes were associated with estrogen, cholesterol and lipid metabolism, inflammation, coagulation, and vasoreactivity. This pilot study provides the first proof of principle that gene expression in blood cells correlates with CIMT. These results point to potential pathophysiological mechanisms underlying sex differences in stroke risk. Since the sample size is small, the findings are preliminary and need to be confirmed in independent, larger studies.
Keywords: Carotid intima–media thickness, Sex differences, Stroke care, Gene expression profiling, Atherosclerosis
Introduction
Gender modulates stroke risk. While stroke incidence is higher in men, women suffer more devastating outcomes [1, 2]. Men are more likely to have coronary artery disease, peripheral artery disease, and smoke tobacco, whereas women are more likely to have hypertension and atrial fibrillation [3–5]. Screening for sub-clinical disease, such as carotid intima–media thickness (CIMT), can be used to identify those patients at risk, despite having low Framingham Risk Profile scores [6]. Identifying differences in stroke risk profiles between men and women will help in developing gender-specific primary prevention strategies.
The association between CIMT and cardiovascular disease is well established, with CIMT being a strong predictor of future stroke [7]. CIMT is an earlier manifestation of atherosclerosis than coronary artery calcification as it can be measured prior to the development of calcified atherosclerotic plaque [8]. The measurement of CIMT by B-mode ultrasonography can detect the extent of atherosclerotic lesion progression and plaque size [9]. Indeed, increased CIMT is more common in patients with acute ischemic stroke compared to controls. Since individuals with a higher CIMT are at a four- to fivefold greater risk of heart attack and stroke [10], it can be used for cardiovascular risk stratification of asymptomatic patients and the primary prevention of stroke [9].
SNPs in specific genes [11] as well as the alleles in the APOE gene [12, 13] have been correlated with CIMT. Other studies have identified genes that distinguished patients with/without coronary disease on angiography [14]. However, studies of the relationship of blood gene expression profiles with CIMT have not been performed. Elucidating gender-specific gene expression profiles associated with the extent of CIMT could help identify gender-specific molecular targets for new interventions to reduce stroke risk.
Materials and Methods
Patient Recruitment
The study was approved by the Wake Forest University Health Sciences (WFUHS) Institutional Review Board. Men and women aged 45–64 years with at least one cardiovascular risk factor (type II diabetes mellitus, hypertension, hypercholesterolemia, tobacco smoking, or metabolic syndrome) were recruited as part of the Sex Age and Variation in Vascular functionalitY (SAVVY) study (NCT00681681) [15]. Exclusion criteria included past history of cardiovascular disease, BMI>46 kg/m2, history of cancer, chronic infection, autoimmune disease, or blood dyscrasias. Subjects were recruited from the community using one of three methods. (1) At community stroke and heart health screenings, the screening nurse measured blood pressure, cholesterol, and blood glucose, as well as a screen for transient ischemic attack (TIA)/stroke symptoms, and other vascular diseases. Based on the age and risk factors identified, the nurse asks the individual if he or she is interested in being contacted about the SAVVY study. (2) In primary care clinics not affiliated with WFUHS, the provider distributes the study brochure to eligible patients. (3) At community lectures about stroke, the investigator (CB) described the study and handed out study brochures. All participants signed informed consent prior to enrollment. Eligible subjects (n=52) underwent CIMT measurement and venipuncture after an overnight fast.
CIMT
CIMT measurements were performed by two certified sonographers and ultrasound readers at WFUHS. B-mode ultrasound was performed with the Acuson Sequoia C512 Ultrasound System and a 8 MHz L5 transducer. Two consecutive defined angles were used to select images of the near and far walls of the common, internal, and bifurcation of the carotid arteries. Using the flow divider between the internal and external carotid arteries as the reference point, the carotid artery is divided into 10-mm segments (bifurcation is 10 mm proximal to the flow divider, common carotid is 10–20 mm proximal, and internal carotid is 10 mm distal to the flow divider). The measurements of each segment were obtained over a 5 s clip that includes five cardiac cycles, and stored until evaluated. The mean average CIMT of all 12 segments (near and far wall of six segments bilaterally) was used for assessment of relationships with gene expression.
RNA Isolation
Whole blood was collected via venipuncture into three PAXgene tubes (PreAnalytiX, Hilden, Germany) and processed as previously described [16].
Microarray Hybridization
Biotin-labeled cDNA was synthesized from 50 ng of total RNA and RNA was hybridized on Affymetrix Genome U133 Plus 2.0 GeneChips (Affymetrix, Santa Clara, CA) as described previously [16].
Statistical Analysis
The patient data were analyzed using the Chi-square test. Statistical significance was determined at p<0.05. The patient data is expressed either as percentage and proportion of patients or as mean±SD. Due to the small sample size of this pilot study, multiple comparison corrections were not employed.
Microarray Data and Pathways Analyses
Gene expression analyses were performed as previously described [16]. Pearson’s correlation was used to correlate CIMT with gene expression at p<0.05. Ingenuity pathway analysis (IPA; Ingenuity Systems Inc, Redwood City, CA, USA) was then used to identify biologically significant pathways. A p value cut-off of <0.1 was used to increase the number of genes evaluated per pathway to further support a role for a given pathway, a preferred approach for pathway analyses. A Fisher’s exact test was used to determine whether there were more genes expressed in a given pathway than would be expected by chance (p<0.05).
Results
Patient Characteristics
Baseline characteristics for all patients are shown in Table 1. There were more men that had ever smoked compared to women (p=0.05) and women had more first degree relatives with TIAs (p=0.01). There were non-significant trends for differences between males versus females for CIMT, hypertension, and hypertensive medications. Five women (14.3%) were using hormone therapy at the time of the blood draw.
Table 1.
Abbreviations and acronyms
| APOE | Apolipoprotein E |
| BMI | Body mass index |
| CAMKK2 | Calcium/calmodulin-dependent protein kinase kinase 2 beta |
| CIMT | Carotid intima–media thickness |
| DHCR7 | 7-Dehydrocholesterol reductase |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| ET-1 | Endothelin-1 |
| ETA | Endothelin receptor A |
| ETB | Endothelin receptor B |
| FGF2 | Basic fibroblast growth factor 2 |
| HDL | High-density lipoprotein |
| IL-8 | Interleukin 8 |
| IPA | Ingenuity pathway analysis |
| LDL | Low-density lipoprotein |
| NOS | Nitric oxide synthase |
| PARP | Poly ADP-ribose |
| PLA2 | Phospholipase A2 |
| SAVVY | Sex, Aging and Vascular Variation in functionalitY |
| SNP | Single-nucleotide polymorphism |
| SYMPK | Symplekin |
| VEGFB | Vascular endothelial growth factor B |
RNA Expression in Blood
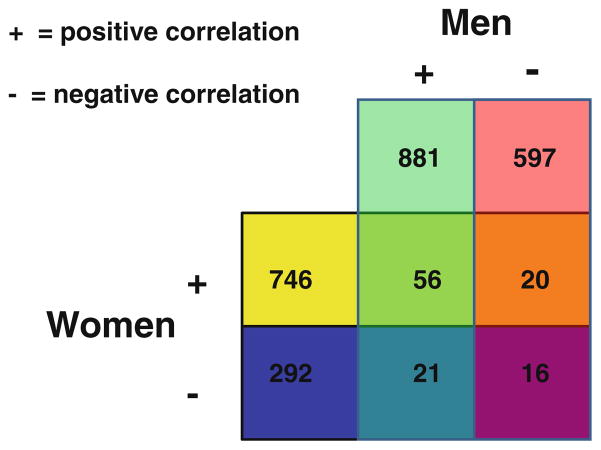
Gender affected the relationships between CIMT and gene expression in blood. CIMT in men correlated positively with 881 genes and negatively with 597 genes (negatively; p<0.05; Fig. 1). CIMT in women correlated positively with 746 genes and negatively with 292 genes (p<0.05; Fig. 1). The top positively correlated genes were BRD8, OCRL, GLT25D1, KIAA2026, and interleukin (IL)-17D in women and C1orf174, FRMD4A, LOC100133233, NMRAL1, and UBE2D2 in men (Supplementary Table 1, 2). The top negatively correlated annotated genes were FOXO1, DNMIP33, HAGHL, ZNF107, and SLC7AI in women and PPP2R2A, EMILIN2, CRNKL1, CSRNP2, and C20orf111 in men (Supplementary Tables 3 and 4).
Fig. 1.
Numbers of genes whose expression was positively (+) or negatively (−) correlated with CIMT in blood of men and women with at least one risk factor for cardiovascular disease but who had no history of cardiovascular disease
Expression levels of numerous genes were similarly correlated with CIMT in men and women, with 56 genes being positively correlated in men and women and 16 genes being negatively correlated in both men and women (p<0.05, Fig. 1; Supplementary Tables 5 and 6). Such genes included sterol carrier protein 2, high-density lipoprotein-binding protein and signal transducer and activator of transcription 5B. These commonly regulated genes were associated with androgen, estrogen, glycosphingolipid, and other pathways (Supplementary Tables 7 and 8).
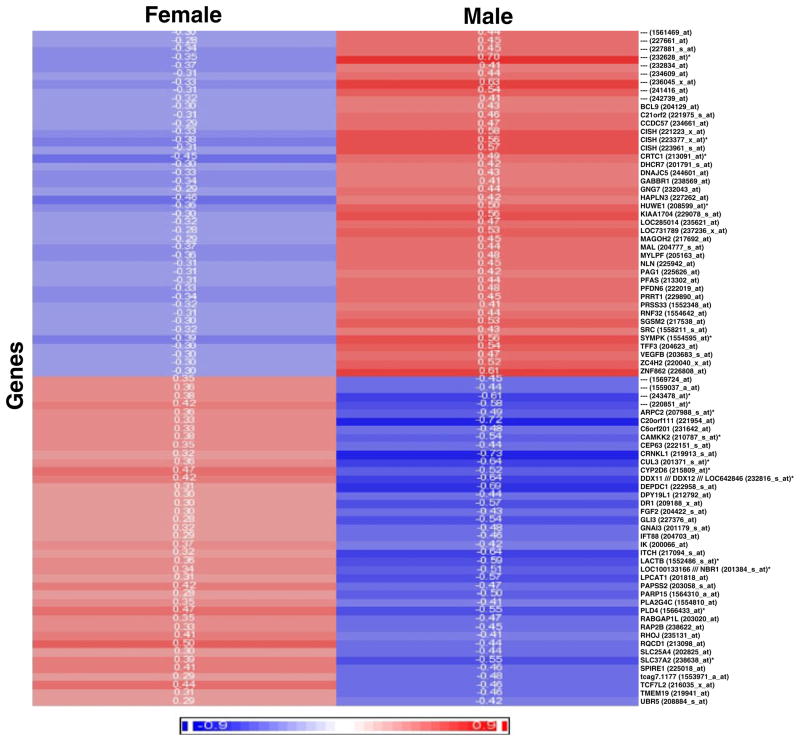
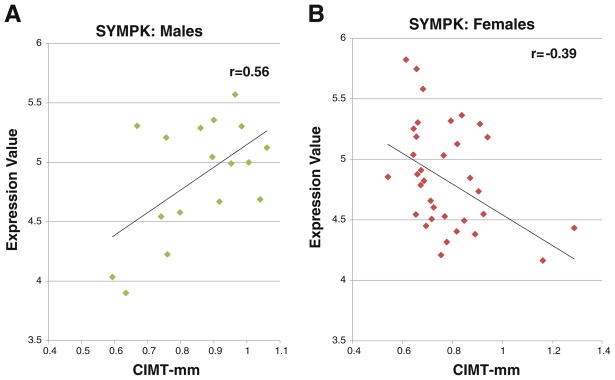
Finally, a small number of genes correlated with CIMT in women in an opposite direction from that observed in men. That is, 20 genes were positively correlated with CIMT in women but negatively correlated with CIMT in men (Fig. 1); and 21 genes positively correlated with CIMT in men but negatively correlated with CIMT in women (p<0.05). There were a total of 41 of these genes at p<0.05 (Fig. 1); and a total of 82 genes at p<0.1 (Fig. 2). A cluster analysis of the 82 genes that correlated with CIMT in opposite directions for men versus women is shown in Fig. 2 (positive correlations in red and negative correlations in blue). Figure 3 shows one example of a gene whose expression correlated with CIMT in opposite directions in men and women. Symplekin regulates polyadenylation machinery components in the post-translational modification process [17].
Fig. 2.
Heat map showing the Pearson correlation coefficients (r values, color coded) for all 82 genes (Y-axis) whose expression was inversely correlated with CIMT (at p<0.1) in females (left column) compared to males (right column). Half of these genes had a p<0.05. The r values for genes whose expression positively correlated with CIMT are shown in shades of red, and the r values for genes whose expression negatively correlated with CIMT are shown in shades of blue. The r values themselves are shown for each gene for females and males
Fig. 3.
An example of a gene whose expression (Y-axis) was inversely correlated with CIMT (X-axis) in males (a) compared to females (b). The r value for males was +0.56 (positive correlation) and the r value for females was −0.39 (negative correlation)
Pathway Analysis
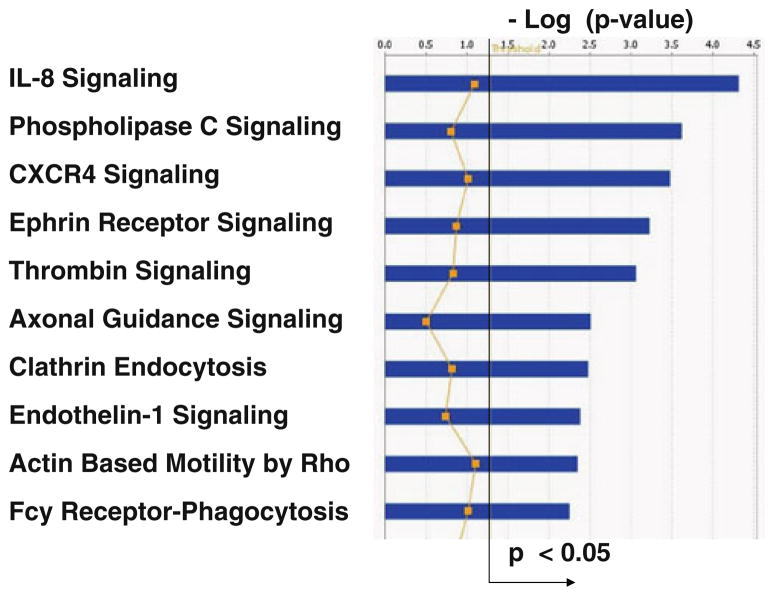
IPA of the genes that are inversely correlated with CIMT in men and women (p<0.10) showed the top canonical pathways to be IL-8 signaling, phospholipase C (PLC) signaling, CXC chemokine receptor 4 (CXCR4) signaling, ephrin receptor signaling, and thrombin signaling, amongst others (Fig. 4; p<0.05; Supplementary Table 9). Inflammatory response and cardiovascular disease were amongst the top diseases/disorders associated with the differentially correlated genes (p<0.05).
Fig. 4.
Top ten canonical pathways (Y-axis) significantly associated with the 82 genes whose expression was inversely correlated with CIMT in women compared to men. The X-axis shows the −log (p value) and the straight black line shows threshold for significance (p<0.05). The zigzag yellow line gives a relative ratio of the number of genes expressed in the pathway divided by the total number of genes in the pathway. The legend for the yellow line is not included
Discussion
This is the first study to demonstrate relationships between gene expression in blood and CIMT. The main findings of the present study were: (1) expression of hundreds of genes correlated with CIMT that were unique to women and men; (2) a number of genes correlated similarly in both men and women; and (3) a smaller set of genes correlated with CIMT in men and women, but in opposite directions. Given the large number of genes and pathways, this report will focus on the latter group of genes that inversely correlated with CIMT in men and women since many of these genes were linked to estrogen pathways. This would have particular importance for the age-related and hormonal changes that occur in both women and men and to hormone therapies in women.
Estrogen-Related Genes
Of the genes that correlated in opposite directions in women compared to men, at least nine were associated with or regulated by estrogen. These included 7-dehydrocholesterol reductase (DHCR7), GNG7, VEGFB, calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2), fibroblast growth factor 2 (FGF2), LPCAT1, PAPSS, PARP15, and phospholipase A2, group IVC (PLA2G4C). DHCR7 catalyzes the conversion of 7-dehydrocholesterol to cholesterol [18]. DHCR7 is upregulated in response to colony-stimulating factor-1, leading to an increase in free cholesterol levels, contributing to atherosclerotic lesions, and plaque progression, and thereby providing a link between inflammation and cardiovascular disease. PLA2G4C cleaves membrane phospholipids, releasing fatty acids, and lysophospholipids, which are second messengers and lipid mediators [19]. Application of PLA2 to endothelial cell cultures increases the expression of adhesion molecules including intercellular adhesion molecule-1, vascular cell adhesion molecule-1, platelet-endothelial cell adhesion molecule-1, P-selectin, and E-selectin on endothelial cells [19]. PLA2 phospholipases are pro-atherogenic, with increased levels detected within the necrotic core of advanced atherosclerotic lesions compared to less-advanced lesions [20]. In addition, circulating levels of LPA2 family member Lp-LPA2 have been associated with increased stroke risk among hormone therapy non-users in the Hormones and Biomarkers Predicting Stroke Study, a nested case control subset of the Women’s Health Initiative [20]. CAMKK2 is involved in macrophage apoptosis in atherosclerotic lesions [21] and ATP-dependent activation of ERK1/2 in vascular smooth muscle cells. Transcriptional profiling of right ventricular outflow tract has identified CAMKK2 as being upregulated in patients with idiopathic ventricular arrhythmias relative to normal controls, with obvious implications for stroke, although potential sexual dimorphism in this relationship was not specifically evaluated in this study [22]. Sexual dimorphism in the role of CAMKK2 has been observed in brain function (memory and hippocampal gene expression) in mice hypomorphic for CAMKK2 expression [23]. CAMKK2 is also important in signaling of adiponectin through the AdipoR1 receptor [24]. FGF2, negatively correlated in men yet positively correlated in women, is involved in the formation of new blood vessels, tissue development, and remodeling and modulates ischemia/reperfusion injury, myocardial infarction, vascular remodeling, and control of vascular tone. Increased FGF2 is associated with chronic inflammatory diseases, such as atherosclerosis. Activated leukocytes in early atherosclerosis release FGF2, triggering a cascade that results in vascular smooth muscle proliferation and migration, and intima hyperplasia [25]. Anti-FGF2 antibodies reduce VSMC proliferation after arterial injury [26]. Indeed, FGF2 levels are higher in patients with atherosclerosis, with levels of FGF2 correlating with the presence of risk factors for atheroma formation. The expression of FGF2 is regulated by a number of factors including HDL which decreases production and release [26] and estrogen which increases expression [27, 28]. VEGF mediates smooth muscle cell proliferation and migration, and influences vascular permeability, leukocyte recruitment, and apoptosis [29]. Notably, inhibition of VEGF leads to a reduction in atherosclerosis [29].
Vascular-Related Genes
In addition to estrogen-related genes, a number of vascular-related genes were inversely correlated with CIMT in women compared to men including IL-8 signaling, thrombin signaling, and endothelin-1 (ET-1) signaling. IL-8 is a mediator of the inflammatory response, serving as a chemo-attractant for neutrophils and some T lymphocytes, modulating monocyte-endothelial cell interactions under flow conditions and influencing angiogenesis. IL-8 expression by human aortic smooth muscle dramatically increases in senescent cell cultures and plays a role in the progression of atherosclerosis and vascular calcification [30]. IL-8 levels in endarterectomized carotid plaques are lower in women compared to men [31]. Thrombin cleaves fibrinogen to form fibrin and also activates factors V, VII, VIII, and XIII, as well as protein C. Accordingly, thrombin is involved in blood clotting, inflammation, and wound healing. ET-1 is produced by vascular endothelial cells, epithelial cells, and microglia, where it modulates vascular tone. ET-1 maintains vascular tone by binding to endothelin A (ETA) and endothelin B (ETB) receptors. ET-1 receptor blockade decreases atherosclerosis by normalizing endothelial dysfunction and decreasing atheroma formation [32]. ET-1 positively correlates with total cholesterol and low-density lipoprotein (LDL) cholesterol in patients with a history of hypertension and may be useful in estimating atherosclerotic burden [33]. Ovarian hormones modulate the ETB receptor vascular response in hypertension [34] and regulate the expression of NOS and preproET-1 [35]. Ovariectomy in female rats worsens hypertension and ETB-mediated responses [34] (Table 2).
Table 2.
Demographics of SAVVY subjects
| Characteristic | All subjects (n=52)
|
Males (n=17)
|
Females (n=35)
|
p value |
|---|---|---|---|---|
| % (number) or mean (SD) | % (number) or mean (SD) | % (number) or mean (SD) | ||
| Age (years) | 56.23 (5.4) | 54.47 (5.03) | 57.08 (5.47) | 0.10 |
| Ethnicity | ||||
| Black | 11.53 (6/52) | 5.88 (1/17) | 14.28 (5/35) | 0.37 |
| White | 86.53 (45/52) | 94.11 (16/17) | 82.85 (29/35) | 0.26 |
| Hispanic | 0.00 (0/52) | 0.00 (0/17) | 0.00 (0/35) | 0.00 |
| Other | 1.92 (1/52) | 0.00 (0/17) | 28.57 (1/35) | 0.48 |
| Ever smoker | 32.7 (18/52) | 52.94 (9/17) | 25.71 (9/35) | 0.05 |
| Hypertension | 53.85 (28/52) | 70.58 (12/17) | 45.71 (16/35) | 0.09 |
| Hypercholesterolemia | 80.77 (42/52) | 70.58 (12/17) | 85.71 (30/35) | 0.19 |
| Diabetes | 19.23 (10/52) | 23.52 (4/17) | 17.14 (6/35) | 0.58 |
| Atrial fibrillation | 0.00 (0/52) | 0.00 (0/17) | 0.00 (0/35) | 1.00 |
| Exercise | 50 (26/52) | 52.94 (9/17) | 48.57 (17/35) | 0.76 |
| BMI | 29.9 (4.99) | 30.44 (4.63) | 29.74 (5.11) | 0.63 |
| Mean avgerage CIMT (mm) | 0.80 (0.15) | 0.85 (0.14) | 0.77 (0.15) | 0.09 |
| Antihypertensive medication | 51.92 (27/52) | 70.58 (12/17) | 42.85 (15/35) | 0.06 |
| Lipid-lowering medication | 40.38 (21/52) | 47.05 (8/17) | 37.14 (13/35) | 0.49 |
| Diabetes medication | 19.23 (10/52) | 23.52 (4/17) | 17.14 (6/35) | 0.58 |
| Cardiovascular medication | 1.92 (1/52) | 0.00 (0/17) | 2.85 (1/35) | 0.48 |
| Anti-thrombotic medication | 1.92 (1/52) | 5.88 (1/17) | 0.00 (0/35) | 0.14 |
| Family history of stroke | 40.38 (21/52) | 29.41 (5/17) | 45.71 (16/35) | 0.26 |
| FDR hypertension | 84.61 (44/52) | 82.23 (14/17) | 85.71 (30/35) | 0.75 |
| FDR diabetes | 61.53 (32/49) | 62.5 (10/16) | 66.66 (22/33) | 0.77 |
| FDR hypercholesterolemia | 88.37 (38/43) | 84.61 (11/13) | 90.00 (27/30) | 0.61 |
| FDR heart disease | 59.61 (31/52) | 58.82 (10/17) | 65.62 (21/32) | 0.63 |
| FDR TIA (mini-stroke) | 27.08 (13/48) | 0.00 (0/17) | 41.93 (13/31) | 0.01 |
| FDR clot (lung, leg) | 8.69 (4/46) | 13.33 (2/15) | 5.71 (2/31) | 0.39 |
| FDR peripheral artery disease | 8.51 (4/47) | 0.00 (0/16) | 12.90 (4/31) | 0.13 |
FDR first degree relative
Limitations
The small sample size and unequal numbers of male and female subjects and their race–ethnic makeup limits the conclusions that can be made from this study. Though confounding variables were similar in the groups, these need to be included in future analyses. For women, the use of hormone therapy is a potential confounder, but this represented only a small fraction of our subjects, so it was unlikely to have influenced the results. Adjusting for hormone therapy use and endogenous sex steroid hormones will be a focus in future studies. In addition, since a correction for multiple comparisons was not performed many of the genes may represent false positives. However, those genes that correlate in both males and females are not likely to be false positives because they are in effect replicated in two different groups: males and females. Indeed, the only way to be confident of any of the genes and pathways identified in this preliminary study is to replicate them in a separate, independent larger cohort [36, 37].
The use of whole blood makes it impossible to assign expression of a given gene to a specific cell type, and the study by design could not assess gene expression within the carotid arteries. It is not possible to determine from these types of studies whether the gene expression correlations represented a cause or an effect of the disease. It is also important to note that although genes or pathways significantly correlate with CIMT in opposite directions in men and women, this is not the equivalent of a risk factor having opposite effects in by gender. Lastly, the present study represents a pilot from an ongoing larger study and there are no previous reports for comparison.
Conclusions
We observed differential correlations of CIMT with gene expression in men and women at risk of stroke, identifying genes involved in pathways central to cholesterol/lipid metabolism, IL-8, thrombin, and endothelin signaling. These genes/pathways may contribute to the different clinical stroke risk profiles observed in men and women, and may represent targets for understanding pathophysiology of gender differences in stroke risk.
Supplementary Material
Acknowledgments
We thank Ryan Davis and Jeff Gregg of the UC Davis Genomics Core for processing the microarrays. We also thank the many individuals in the Sharp group who helped teach the skills needed to do the analyses.
Sources of Funding RJT is supported by a National Health and Medical Research Council (Australia; 519365) Postdoctoral fellowship. CDB and the SAVVY study are supported by NIH/NINDS K02 NS058760. FRS is supported by NIH/NINDS-NS056302 and the American Heart Association Bugher Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12975-011-0066-4) contains supplementary material, which is available to authorized users.
Conflict of Interests None.
Contributor Information
Renée J. Turner, Department of Neurology and M.I.N.D. Institute, University of California at Davis, Sacramento, CA 95817, USA
Cheryl D. Bushnell, Email: cbushnel@wfubmc.edu, Department of Neurology, Wake Forest University Health Sciences, Medical Center Boulevard, Winston-Salem, NC 27157, USA
Thomas C. Register, Department of Pathology, Section on Comparative Medicine, Wake Forest University Health Sciences, Winston-Salem, NC 27157, USA
Frank R. Sharp, Department of Neurology and M.I.N.D. Institute, University of California at Davis, Sacramento, CA 95817, USA
References
- 1.Gargano J, Reeves M. Sex differences in stroke recovery and stroke-specific quality of life. Stroke. 2007;38:2541–8. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 2.Reeves M, Bushnell C, Howard G, Gargano J, Duncan P, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–26. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapral MK, Fang J, Hill MD, Silver F, Richars J, Jaigobin C, et al. Sex differences in stroke care and outcomes. Results from the registry of the Canadian Stroke Network. Stroke. 2005;36:809–14. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 4.Di Carlo A, Lamassa M, Baldreschi M, Pracucci G, Basile AM, Wolfe CD, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe. Data from a Multicenter Multinational Hospital-Based Registry. Stroke. 2003;34:1114–9. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 5.Smith D, Murphy P, Santos P, Phillips M, Wilde M. Gender differences in the Colorado Stroke Registry. Stroke. 2009;40:1078–81. doi: 10.1161/STROKEAHA.108.541730. [DOI] [PubMed] [Google Scholar]
- 6.Grewal J, Anand S, Islam S, Lonn E. Prevalence and predictors of subclinical atherosclerosis among asymptomatic “low risk” individuals in a multiethnic population. Atherosclerosis. 2008;197:435–42. doi: 10.1016/j.atherosclerosis.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Folsom A, Kronmal R, Detrano R, O’Leary D, Bild D, Bluemke D, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence. The Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Jones D, Adams R, Brown T, Carnethon M, Dai S, De Simone G, et al. Heart Disease and Stroke Statistics 2010 Update. A report from the American Heart Association. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 9.de Groot E, van Leuven S, Duivenvoorden R, Meuwese M, Akdim F, Bots M, et al. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5:280–8. doi: 10.1038/ncpcardio1163. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary D, Polak J, Kronmal R, Manolio T, Burke GL, Wolfson S. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 11.Lanktree M, Hegele R, Yusuf S, Anand S. Multi-ethnic genetic association study of carotid intima-media thickness using a targeted cardiovascular SNP microarray. Stroke. 2009;40:3173–9. doi: 10.1161/STROKEAHA.109.556563. [DOI] [PubMed] [Google Scholar]
- 12.Elosua R, Ordovas J, Cupples L, Fox C, Polak J, Wolf P, et al. Association of APOE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J Lipid Res. 2004;45:1868–75. doi: 10.1194/jlr.M400114-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Paternoster L, Anani N, Gonzalez M, Lewis S, Sudlow C. Association between apolipoprotein E genotype and carotid intima-media thickness may suggest a specific effect on large artery atherothrombotic stroke. Stroke. 2008;39:48–54. doi: 10.1161/STROKEAHA.107.488866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wingrove J, Daniels S, Sehnert A, Tingley W, Elashoff M, Rosenberg S, et al. Correlation of peripheral blood gene expression with the extent of coronary artery stenosis. Circulation Cardiovascular Genetics. 2008;1:31–8. doi: 10.1161/CIRCGENETICS.108.782730. [DOI] [PubMed] [Google Scholar]
- 15.Sex Differences in Vascular Markers of Stroke Risk (SAVVY) [database on the Internet] U.S. National Institutes of Health; 2008. [cited November 27, 2010] [Google Scholar]
- 16.Xu H, Tang Y, Liu D-Z, Ran R, Ander B, Apperson M, et al. Gene expression in peripheral blood differs after cardioembolic compared with large-vessel atherosclerotic stroke: biomarkers for the etiology of ischemic stroke. J Cerebral Blood Flow and Metabolism. 2008 doi: 10.1038/jcbfm.2008.22. [DOI] [PubMed] [Google Scholar]
- 17.Mandel C, Bai Y, Tong L. Protein factors in pre-mRNA 3′-end processing. Cell Mol Life Sci. 2008;65:1099–122. doi: 10.1007/s00018-007-7474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterham H. Defects of cholesterol biosynthesis. FEBS Lett. 2006;580:5442–9. doi: 10.1016/j.febslet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Karabina S, Gora S, Atout R, Ninio E. Extracellular phospholipases in athersclerosis. Biochimie. 2010;92:594–600. doi: 10.1016/j.biochi.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Wasserthiel-Smoller S, Kooperberg C, McGinn A, Kaplan R, Hsia J, Hendrix S, et al. Lipoprotein-associated phospholipase A2, hormone use, and the risk of ischemic stroke in postmenopausal women. Hypertension. 2008;51:1115–22. doi: 10.1161/HYPERTENSIONAHA.107.103721. [DOI] [PubMed] [Google Scholar]
- 21.Tabas I, Seimon T, Timmins J, LI G, Lim W. Macrophage apoptosis in advanced atherosclerosis. Ann NY Acad Sci. 2009;1173 (Suppl 1):E40–5. doi: 10.1111/j.1749-6632.2009.04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasdemir C, Aydin H, Celik H, Simsek E, Payzin S, Kayikcioglu M, et al. Transcriptional profiling of septal wall of the right ventricular outflow tract in patients with idiopathic ventricular arrhythmias. Pacing Clin Electrophysiol. 2010;33:159–67. doi: 10.1111/j.1540-8159.2009.02606.x. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno K, Ris L, Sánchez-Capelo A, Godaux E, Giese K. Ca2+/calmodulin kinase kinase alpha is dispensable for brain development but is required for distinct memories in male, though not in female, mice. Mol Cell Biol. 2006;26:9094–104. doi: 10.1128/MCB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1-alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–9. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 25.Barillari G, Iovane A, Bonuglia M, Albonici L, Garofano P, Di Campli E, et al. Fibroblast growth factor-2 transiently activates the p53 oncosuppressor protein in human primary vascular smooth muscle cells: implications for atherogenesis. Atherosclerosis. 2010;210:400–6. doi: 10.1016/j.atherosclerosis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Cucina A, Scavo M, Muzzoli L, Coluccia P, Ceccarini S, Fuso A, et al. High density lipoproteins downregulate basic fibroblast growth factor production and release in minimally oxidated-LDL treated smooth muscle cells. Atherosclerosis. 2006;189:303–9. doi: 10.1016/j.atherosclerosis.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Liao S, Bodmer J, Pietras D, Azhar M, Doetschman T, Schultz J. Biological functions of the low and high molecular weight protein isoforms of fibroblast growth factor-2 in cardiovascular development and disease. Dev Dyn. 2009;238:249–64. doi: 10.1002/dvdy.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobrino A, Mata M, Laguna-Fernandez A, Novella S, Oviedo P, Garcia-Perez M, et al. Estradiol stimulates vasodilatory and metabolic pathways in cultured human endothelial cells. PLoS ONE. 2009;4:e8242. doi: 10.1371/journal.pone.0008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holm P, Slart R, Zeebregts C, Hillebrands J, Tio R. Atherosclerotic plaque development and instability: a dual role for VEGF. Ann Med. 2009;41:257–64. doi: 10.1080/07853890802516507. [DOI] [PubMed] [Google Scholar]
- 30.Burton D, Giles P, Sheerin A, Smith S, Lawton J, Ostler E, et al. Microarray analysis of senescent vascular smooth muscle cells: a link to atherosclerosis and vascular calcification. Exp Gerontol. 2009;44:659–65. doi: 10.1016/j.exger.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Hellings W, Pasterkamp G, Verhoeven B, De Kleijn D, de Vries J, Seldenrijk C, et al. Differences between men and women in the composition of atherosclerotic plaque, as an explanation for the lower success rate of carotid endarterectomy in women. Ned Tijdschr Geneeskd. 2008;152:2624–31. [PubMed] [Google Scholar]
- 32.Barton M, d’Uscio L, Shaw S, Meyer P, Moreau P, Luscher T. ETA receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension. 1998;31:499–504. doi: 10.1161/01.hyp.31.1.499. [DOI] [PubMed] [Google Scholar]
- 33.Sayama H, Nakamura Y, Saito N, Konoshita M. Does the plasma endothelin-1 concentration reflect atherosclerosis in the elderly? Gerontology. 1999;45:312–6. doi: 10.1159/000022111. [DOI] [PubMed] [Google Scholar]
- 34.David F, Carvalho M, Cobra A, Nigro D, Fortes Z, Reboucas N, et al. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension. 2001;38:692–6. doi: 10.1161/01.hyp.38.3.692. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Barber D, Lewis D, McGregor C, Seick G, Fitzpatrick L, et al. Gender and transcriptional regulation of NO synthase and ET-1 in porcine aortic endothelial cells. Am J Physiol. 1997;273:H1962–7. doi: 10.1152/ajpheart.1997.273.4.H1962. [DOI] [PubMed] [Google Scholar]
- 36.Stamova B, Xu H, Jickling G, Bushnell C, Tian Y, Ander B, et al. Gene expression profiling of blood for the prediction of ischemic stroke. Stroke. 2010;41:2171–7. doi: 10.1161/STROKEAHA.110.588335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jickling G, Xu H, Stamova B, Ander B, Zhan X, Tian Y, et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann Neurol. 2010;68:681–92. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.