Figure 5.
Structure of the CALMANTH(1-264):VAMP8(11-41) Chimera
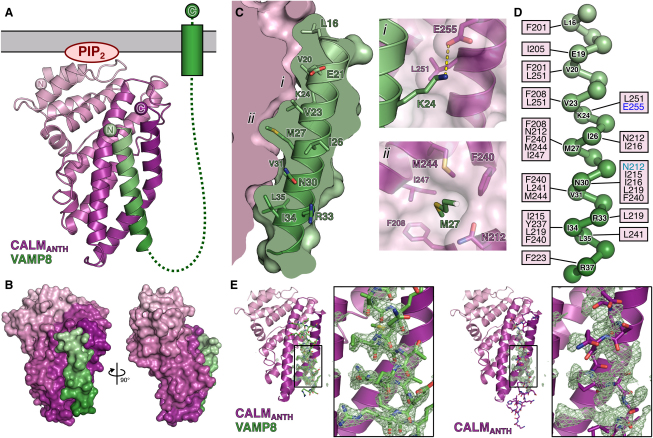
(A) Overall structure of the complex with CALMANTH colored as in Figure 4 with VAMP8 colored from pale (residue 15) to dark (residue 37) green. The relative position of the membrane (gray bar) is inferred from the position of the PtdIns4,5P2 (marked as PIP2) binding site on CALMANTH as in 1HF8. The dotted line is a schematic representation of how the remainder of the cytoplasmic portion of VAMP8 domain connects its CALM binding helix to its transmembrane helix.
(B) Orthogonal views of the CALMANTH:VAMP8 complex shown in molecular surface representation colored as in (A).
(C) Spatial complementarity of the CALMANTH:VAMP8 interface with key side chains involved in the binding of CALMANTH by VAMP8 shown. Molecular details of the interactions of the key residues (i) K24 and (ii) M27 are shown.
(D) Schematic representation of VAMP8 residues 15–38. CALMANTH residues that make hydrophobic interactions with VAMP8 are labeled in black, those that make salt bridge interactions with VAMP8 are labeled in blue and those that make hydrogen bonds with VAMP8 are labeled in turquoise.
(E) The final refined VAMP8 helix (green sticks) is shown in unbiased (FO–FC) electron density contoured at 3.5 σ calculated before the addition of the helix to the model (left panel). The VAMP8 helix lies on the same face as, but differs significantly from, the orientation of helix α11 (purple sticks) in the unliganded CALMANTH structure (right panel).
See also Figure S5.