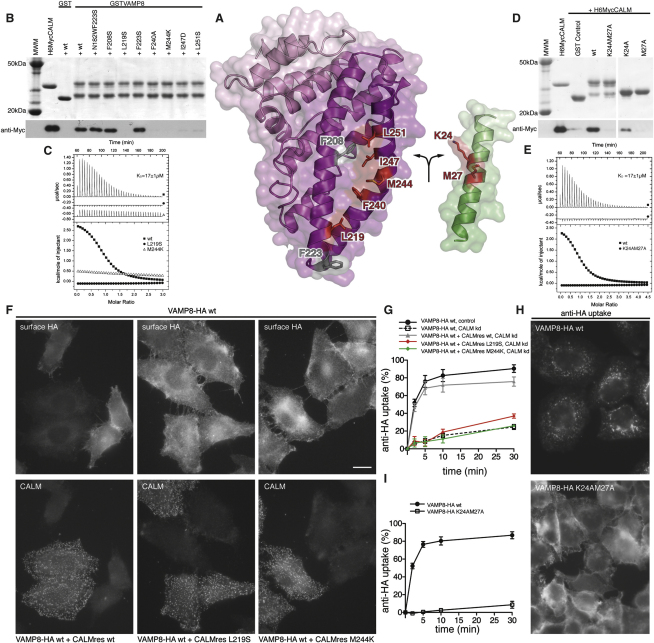
Figure 6.
Mutation of Key Residues in the CALMANTH:VAMP8 Interface Abolishes Their Interaction In Vitro and the Endocytosis of VAMP8 In Vivo
(A) Structure of the CALMANTH:VAMP8 complex “opened out like a book” as indicated by the arrows. Residues whose mutation affect binding between CALM and VAMP8 are colored and labeled in red, while mutations that have no effect are colored and labeled in gray.
(B) Pull-down experiments using GSTVAMP8 and WT or mutant His6MycCALMANTH as indicated. Top panel: Coomassie blue stained gel. Lower panel: western blot probed with anti-myc. The mutations L219S, F240A, M244K, I247D, and L251S in His6MycCALMANTH abolished binding to VAMP8.
(C) ITC quantitating the binding of certain point mutant versions of CALMANTH to VAMP8. The wild-type CALMANTH binds with a KD17 ± 1μM (black squares) whereas CALMANTH L219S (black circles) and M244K (open triangles) showed no measurable interaction with VAMP8. Data for CALMANTH L219S and M244K are translated by 0.3 μcal/s and −0.7 μcal/s respectively.
(D) GST pull-down experiments using His6MycCALMANTH and WT or mutant GSTVAMP8 fusion proteins as indicated. Top panel: Coomassie blue stained gel. Lower panel: western blot probed with anti-myc. Wt GSTVAMP8 bound CALMANTH whereas the K24AM27A VAMP8 did not. The GSTVAMP8 K24A mutant interacted weakly with CALMANTH, however, the single M27A mutation of VAMP8 was sufficient to completely abolish the interaction with CALMANTH.
(E) ITC quantitating the binding of K24AM27A mutant version of VAMP8 to CALMANTH. Wt GSTVAMP8 bound wt CALMANTH with a KD17 ± 1μM (black squares) whereas GSTVAMP8 K24AM27A (black circles) showed no measurable interaction. Data for GSTVAMP8 K24AM27A is translated by −0.3 μcal/s for clarity.
(F) Cells expressing either VAMP8-HA alone, or VAMP8-HA plus Myc-tagged siRNA-resistant CALM (CALMres: wt, L219S, or M244K) were mixed together and endogenous CALM was depleted by siRNA treatment. The cells were then fixed without permeabilization and labeled with anti-HA antibody, and then permeabilized and labeled with anti-CALM. The scale bar represents 20 μm.
(G) Endocytosis of anti-HA in cells coexpressing VAMP8-HA and siRNA-resistant wild-type, L219S or M244K mutant myc-tagged CALM. Antibody was bound to the cells at 4°C, then the cells were warmed to 37°C for 2–30 min and antibody remaining at the cell surface was quantified by flow cytometry. Each point is derived from at least 3 separate experiments; the error bars show the SEM. Expression of the CALM construct rescues the knockdown phenotype, but expression of the two CALM mutants does not.
(H) Localization of anti-HA in cells expressing wild-type or K24AM27A mutant VAMP8-HA. The cells were allowed to endocytose the antibody for 40 min, then processed for immunofluorescence. Unlike the cells expressing wild-type VAMP8, the cells expressing the mutant have retained the antibody on the plasma membrane. The scale bar represents 20 μm.
(I) Endocytosis of anti-HA in cells expressing wild-type or K24AM27A mutant VAMP8-HA, using the flow cytometry assay described in Figure S1B. There is negligible endocytosis of the mutant construct.