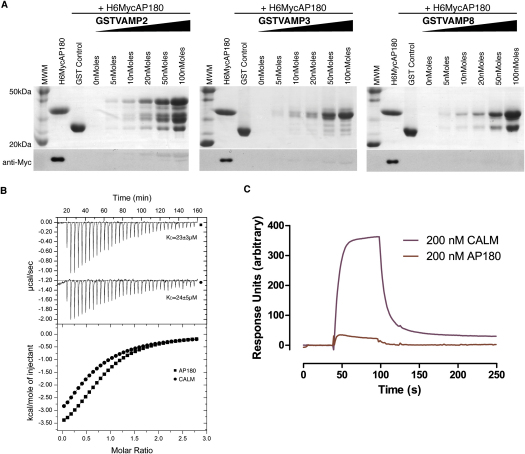
Figure S2.
AP180ANTH Does Not Bind VAMP2, VAMP3, or VAMP8, Related to Figure 2
(A) GST Pull downs using His6MycAP180ANTH and the GST-fusion proteins indicated. Top panel: Coomassie blue stained gel. Lower panel: western blot probed with anti-myc. The lane adjacent to the molecular weight markers (MWM) is loaded with His6MycAP180ANTH only. The ANTH domain of AP180 does not bind to VAMP2, 3, and 8.
(B) Isothermal titration calorimetry of the binding of CALMANTH and AP180ANTH to Ins(1,4,5)P3 gave comparable binding affinities (KD24 ± 5μM and KD23 ± 3μM respectively) confirming both proteins were folded and functionally active. Data for CALM is translated by −1.2 μcal/s for clarity.
(C) Surface Plasmon Resonance of CALMANTH and AP180ANTH also showed AP180ANTH did not bind to GST VAMP8. The SNARE was coupled directly to a CM5 chip and the binding of the ANTH domain proteins was assayed by flowing 200 nM analyte (CALMANTH or AP180ANTH) across the chip for 60 s at a flow rate of 30μl/min in 20 mM HEPES (pH 7.4), 150 mM NaCl, 4 mM DTT. Data were recorded on a Biacore 3000 (GE Healthcare) at 20°C and the binding of analyte to a mock channel (GST) has been subtracted.