Figure 2.
The 2.2 Å Crystal Structure of the BUBR1-Blinkin Complex Reveals an Unexpected Mode of Binding
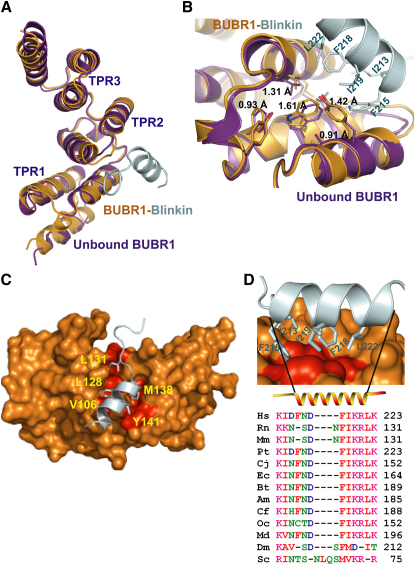
(A) Superposition of the free (magenta) and Blinkin-bound TPR BUBR1 crystal structures (orange) reveals that little conformational changes occur upon ligand binding. A closeup of TPR2 BUBR1, where the slight conformational changes that result from Blinkin binding are most noticeable.
(B) Surface representation of TPR BUBR1; the hydrophobic residues relevant for the interaction of this protein with Blinkin (blue ribbon) are highlighted in red.
(C) Blinkin residues I213, F215, F218 and I219 bind a shallow hydrophobic groove.
(D) Amino acid sequence alignment of the Blinkin BUBR1 binding region reveals a high conservation of these residues in higher organisms. Each molecule representation was generated with Pymol (DeLano, 2002). The aligned sequences are from Homo sapiens (Hs); Rattus norvegicus (Rn); Mus musculus (Mm); Pan troglodytes (Pt); Callithrix jacchus (Cj); Equus caballus (Ec); Bos taurus (Bt); Ailuropoda melanoleuca (Am); Canis familiaris (Cf); Oryctolagus cuniculus (Oc) and Monodelphis domestica (Md); Drosophila melanogaster (Dm); and Saccharomyces cerevisiae (Sc).