Abstract
It is now generally accepted that Polynesia was first settled by peoples from southeast Asia. An alternative that eastern parts of Polynesia were first inhabited by Amerindians has found little support. There are, however, many indications of a ‘prehistoric’ (i.e. before Polynesia was discovered by Europeans) contact between Polynesia and the Americas, but genetic evidence of a prehistoric Amerindian contribution to the Polynesian gene pool has been lacking. We recently carried out genomic HLA (human leucocyte antigen) typing as well as typing for mitochondrial DNA (mtDNA) and Y chromosome markers of blood samples collected in 1971 and 2008 from reputedly non-admixed Easter Islanders. All individuals carried HLA alleles and mtDNA types previously found in Polynesia, and most of the males carried Y chromosome markers of Polynesian origin (a few had European Y chromosome markers), further supporting an initial Polynesian population on Easter Island. The HLA investigations revealed, however, that some individuals also carried HLA alleles which have previously almost only been found in Amerindians. We could trace the introduction of these Amerindian alleles to before the Peruvian slave trades, i.e. before the 1860s, and provide suggestive evidence that they were introduced already in prehistoric time. Our results demonstrate an early Amerindian contribution to the Polynesian gene pool on Easter Island, and illustrate the usefulness of typing for immunogenetic markers such as HLA to complement mtDNA and Y chromosome analyses in anthropological investigations.
Keywords: Polynesia, Amerindians, HLA alleles
1. Introduction
Polynesia (from Greek for many islands) is usually defined as the myriad of islands roughly situated within a triangle with its corners at New Zealand in the southwest, Hawaii in the north and Easter Island in the southeast. It is generally accepted that Polynesia was first settled by peoples from southeast Asia. Exactly where the Polynesians came from has, however, been more controversial (see Friedlander et al. [1] for references). One theory, ‘Entangled Bank’, states that there has been a series of migrations from Asia into the Pacific, making it difficult to exactly identify the ancestors of the Polynesians. At the other end is ‘The Express Train to Polynesia’ theory, which proposes a rapid migration of the ancestors of the Polynesians from the vicinity of Taiwan, without extensive contacts with near Oceanic populations along the way. Studies of maternally inherited mitochondrial DNA (mtDNA) in the area generally support this theory [2,3].
More recent analysis of mtDNA, Y-chromosomal DNA and autosomal genetic markers, including HLA (human leucocyte antigen) genetic markers, as well as linguistic and archaeological evidence suggest a more intermediate model for the migration to Polynesia [1,4], i.e. a ‘ Slow Boat to Polynesia’ theory [5]. This theory also suggests that the ancestors of Polynesians came from the vicinity of Taiwan or southern China, starting their migration approximately 5500 years before present (BP), and first arriving in Melanesia, i.e. the many islands surrounding Australia to the north and east, including the Bismarck Archipelago. Here, they mixed with some local people while developing elements of the Lapita culture, before they migrated eastward reaching Tonga and finally Samoa approximately 3000 BP. From here, they migrated further by inter-island water crossings in their canoes to many islands of the Polynesian archipelago, reaching Easter Island approximately AD 1000 [6] or 1200 [7].
There is, however, also evidence of an early, ‘prehistoric’ (i.e. before Polynesia was discovered by Europeans) South American contact with eastern Polynesia, such as the presence of the Andean sweet potato [8,9] and the bottle gourd [10], which were grown on Easter Island long before it was discovered by Europeans in 1722 [9]. Further, similarities between some old fishing gear from eastern Polynesia and on the coast of northern Chile also suggest early contacts [11], as well as some linguistic and other evidence [12,13]. Some of this evidence as well as the similarities between the giant stone statues (moai) and their platforms (ahu) on Easter Island (and some other Polynesian islands) with pre-inca stone statues and platforms in Tiahuanaco at Lake Titicaca in Bolivia, led Thor Heyerdahl to propose that eastern Polynesia, including Easter Island, was first populated by Amerindians [14]. So far, however, no studies have been able to demonstrate an early Amerindian contribution to the gene pool of Polynesia [15]. The presence of some native American Y chromosomes on Rapa, another island in Polynesia, was assumed to be the result of the repatriation following the Peruvian slave trades in Polynesia in the 1860s, which resulted in an admixture of Amerindian and European genes in the area [16].
2. Previous genetic investigations on Easter Island
During the first expedition of Heyerdahl to Easter Island in 1955–1956, blood samples were collected from some of the inhabitants and typed for a limited number of blood group antigens. However, no proof of a close genetic relationship with Amerindians could be established (references in Thorsby et al. [17]).
In 1971, the 5th International Histocompatibility Workshop decided to use HLA typing in anthropological studies of various populations around the world. We collected blood samples from 69 Easter Islanders, where no foreign admixture was known. The selection of the studied population and their genealogy, based on medical and historical records, has been described in detail elsewhere [17]. Typing was carried out for the very few HLA antigens known at that time, using serological methods having a very low resolution. Thus, only some very ‘broad’ HLA-A and -B antigens could be determined. The data obtained, also including typing for some blood groups and serum types, did not convincingly reveal an early contribution of Amerindian genes to Easter Island [17]. The remaining sera from these investigations were, however, stored in liquid nitrogen.
In 1994, Hagelberg et al. [18] carried out typing of mtDNA markers of specimens obtained from prehistoric skeletal material from the island. The results showed typical Polynesian mtDNA markers. More recent investigations of Alu insertion polymorphisms in chromosomes from native Easter Island individuals also failed to show prehistoric contacts between Easter Island and native Americans [19]. Thus, previous genetic investigations on Easter Island have not been able to reveal an early, prehistoric Amerindian contribution to the Polynesian gene pool of the island.
3. Genetic traces of Amerindians
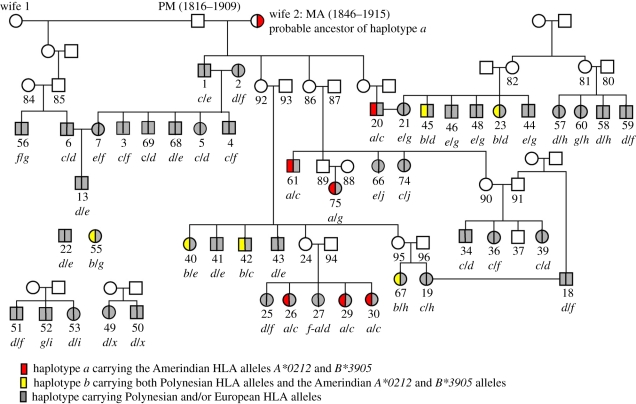
In 2005–2006, we thawed the stored serum samples from our 1971 investigations and were able to extract and amplify DNA from 48 of the samples. The genealogy of the studied individuals, as it had been established in 1971, had to be slightly modified on the basis of new information from Langdon [20] and McCall [21]. The revised genealogy is shown in figure 1. It can be seen that most of the investigated individuals belong to one large family, descending from Pacomio Maori (PM; 1816–1909) and his two wives. We cannot completely exclude that further revisions of the genealogy depicted in figure 1 may be necessary. The genomic HLA data (see later) are, however, fully compatible with the genealogy given in figure 1. The DNA was typed for mtDNA and Y chromosome markers as well as for all known HLA alleles using high-resolution sequencing. Detailed results of these investigations have been reported elsewhere [22], also containing relevant references. A short summary is given here.
Figure 1.
Genealogy of the Easter Island individuals included in our molecular genetic studies of the serum samples from 1971. The numbers of the individuals are the same as used in Lie et al. [22], whereas the haplotype designations are slightly different. The most probable HLA haplotypes (table 1) are listed under each genotyped individual. The two haplotypes carrying Amerindian HLA alleles, haplotypes a and b, are coloured red and yellow, respectively. Haplotypes carrying European (i.e. haplotype c) or Polynesian HLA alleles are coloured grey. Open circles or squares mean that the individuals have not been investigated.
(a). Mitochondrial DNA data
Nineteen unrelated or distantly related individuals were sequenced for the hypervariable control region and the 9-bp deletion of mtDNA. Seventeen individuals carried the previously described full ‘Polynesian motif’. Two individuals carried a slightly different motif, with an A to G transition at position 16247, also seen in Polynesia. These results support a Polynesian origin of the population of Easter Island. No traces of an Amerindian contribution could be detected.
(b). Y chromosome data
All males (n = 26) were genotyped for biallelic Y chromosome markers and some short tandem repeat (STR) polymorphisms. Most of the men had the C to T transition that characterizes haplogroup C-M208, abundant among Polynesian men. Five of the men (no. 1 and his male offspring nos. 3, 4, 68 and 69; figure 1) belonged to the R-M173 haplogroup typical of Europeans and had a Y-STR haplotype found in middle and southern Europe. These results provide further support of a Polynesian origin of the population of Easter Island, but also demonstrate a European contribution to the gene pool (see §3c(ii)). Again, no traces of an Amerindian contribution could be detected.
(c). Genomic HLA data
The results of high-resolution HLA genotyping of the studied population are given in table 1.1 A total of 11 different HLA haplotypes (i.e. particular combinations of the closely linked HLA alleles in the HLA complex) were found (see Lie et al. [22] for how they were established). The probable origin of these haplotypes is given in table 1.
Table 1.
HLA haplotypes (i.e. the particular combination of HLA alleles in the HLA complex on a given chromosome 6) observed in the serum samples from the Easter Islanders collected in 1971.
| haplotypea | HLA-A* | HLA-C* | HLA-B* | HLA-DRB1* | HLA-DQA1* | HLA-DQB1* | probable origin |
|---|---|---|---|---|---|---|---|
| a | 02:12 | 07:02:01 | 39:05 | 08:02:01 | 04:01 | 04:02 | Amerindian |
| b | 02:12 | 07:02:01 | 39:05 | 04:03:01 | 03 | 03:02 | Amerindian (ACB); Amerindian/Polynesian (DRDQ) |
| c | 29:02:01 | 16:01:01 | 44:03:01 | 09:01:02 | 03 | 03:02 | European (ACB); European/Polynesian (DRDQ) |
| d | 24:02:01 | 04:01 | 40:01 | 11:01:01 | 05:01 | 03:01 | Polynesian |
| e | 11:01:01 | 01:02 | 56:02 | 09:01:02 | 03 | 03:03 | Polynesian |
| f | 11:01:01 | 04:03 | 40:10 | 04:03:01 | 03 | 03:02 | Polynesian |
| f-ab | 11:01:01 | 04:03 | 40:10 | 08:02:01 | 04:01 | 04:02 | Polynesian (ACB); Amerindian (DRDQ) |
| g | 24:02:01 | 04:01 | 40:01 | 04:03:01 | 03 | 03:02 | Polynesian |
| h | 02:01 | 01:02 | 55:02 | 12:01:01 | 05:01 | 03:01 | Polynesian |
| i | 24:02:01 | 07:02 | 39:01 | 14:01 | 01:01 | 05:02 | Polynesian |
| j | 24:02 | 08:01 | 48:01 | 08:03:02 | 01:03 | 06:01 | Polynesian |
aThe letters in the first column refer to the HLA haplotypes found and relate to the letters given underneath each genotyped individual in figure 1. Note that the haplotype designations differ slightly from the designations used in Lie et al. [22].
bIndividual 27 (figure 1) has received a recombinant HLA haplotype from her father (no. 94), where the ACB part is from haplotype f, while the DRDQ part is from haplotype a.
(i). Polynesian HLA alleles
All investigated Easter Islanders carried HLA alleles previously observed in Polynesia ([23–25] and www.pypop.org/popdata, which reports the distribution of HLA alleles in 497 populations, based on more than 66 800 individuals investigated). The alleles included some which are characteristic of southeast Asian and Oceanian populations, such as B*40:01, B*55:02, B*56:02, DRB1*08:03, DRB1*14:01 and DQB1*05:02. With the exception of HLA haplotype a (which may be an Amerindian haplotype; see §3c(iii)), all detected haplotypes carry HLA alleles which have previously been observed in Polynesia. Thus, the results of the HLA analysis are also in line with a mainly Polynesian origin of the population of Easter Island.
(ii). European HLA alleles
One HLA haplotype, c, carried HLA alleles in its ACB part which are typical of Southern Europeans, i.e. A*29:02:01-C*16:01:01-B*44:03:01 [25]. However, haplotype c also carried DRB1, DQA1 and DQB1 alleles that are found in many different populations.
PM is the likely source of haplotype c in the studied individuals. This is supported by the fact that some descendants of PM with both of his wives carry this haplotype (figure 1 and see below). Since PM was born in approximately 1816, the ACB part of this HLA haplotype was most probably introduced on Easter Island in the early 1800s, or earlier. The European Y chromosome haplogroup R-M173 was also found in individual 1 (the son of PM) and his sons, providing further evidence for a contribution of European genes in this family. The primary source of these European genes may be a European crewmember on a ship visiting Easter Island before the early 1800s. The particular haplotypic combination of broad A*29 and B*44 alleles (including C*16:01), detected as haplotype A29-B12 by our low resolution serological typing in 1971 [17], has, however, the world highest frequency among Basques (10%; see www.allelefrequencies.net) and is much less frequent in Asia, among Amerindians and in Polynesia. This led Langdon to consider our results to lend further support to his theory that some surviving Basque crewmembers of the Spanish caravel San Lesmes, lost in Polynesia in 1526, may have reached Easter Island before it was discovered by Roggeveen in 1722 [20].
(iii). Amerindian HLA alleles
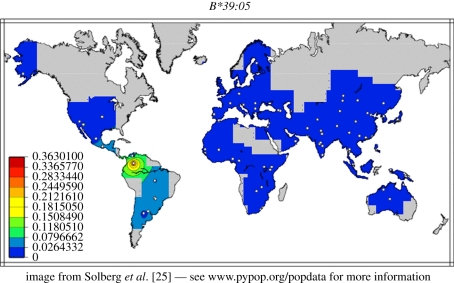
The HLA haplotypes a and b carried the HLA alleles A*02:12 and B*39:05, which have previously almost only been found in Amerindian populations and people of native American ancestry, but which are very rare or absent in Polynesian and other non-Amerindian populations ([25], other references in the literature [22,23]). The distribution of B*39:05 is shown in figure 2.
Figure 2.
The frequency of the HLA allele B*39:05 in various populations [25]. The allele frequencies are given in different colours to the left.
It can be seen that this HLA allele is found in high frequency among natives in South America, and hardly in other populations. A similar distribution is found for A*02:12. Thus, the A*02:12 and B*39:05 alleles are typical Amerindian HLA alleles and may have been generated in America after the migration of an East Asian population via the Bering Strait to America [23].
The other HLA alleles on haplotype a, i.e. C*07:02:01, DRB1*08:02:01, DQA1*04:01 and DQB1*04:02, also have their highest frequencies in Amerindians, but are found too in other populations. Therefore, the particular combination of HLA alleles on haplotype a is likely to be an Amerindian haplotype. Interestingly, a more broadly defined haplotype—A*02-B*39-DRB1*08:02-DQB1*04:02, which therefore might be identical to haplotype a—has previously been found only in Aymara Amerindians from Bolivia, living in the Lake Titicaca area [26]. However, high-resolution typing of the A*02 and B*39 alleles of this Aymara haplotype revealed that it carried the A*02:01 and B*39:06 alleles, respectively (Arnaiz-Villena 2010, personal communication) and not the slightly different A*02:12 and B*39:05 alleles found on haplotype a. Thus, this Aymara Amerindian haplotype is similar, but not identical in its ACB part to the Amerindian haplotype a found on Easter Island.
Haplotype b also carries the Amerindian HLA alleles A*02:12 and B*39:05. The HLA alleles in the DRB1-DQA1-DQB1 part of this haplotype are also found in Amerindians, but are more frequent in Polynesians (see haplotypes f and g; table 1). Thus, haplotype b may be a recombinant Amerindian ACB/Polynesian DRDQ haplotype.
4. When were the Amerindian HLA alleles introduced on Easter Island?
Our investigations cannot answer this question with certainty, except that the Amerindian contribution to the gene pool of Easter Island must have occurred early.
First, we have to establish that these Amerindian alleles did not arrive as a result of the repatriation following the Peruvian slave raids in the 1860s, which resulted in an admixture of Amerindian and European genes in the area [16]. Because the key ancestors of the large family depicted in figure 1, PM and his two wives, were all dead and thus not available for HLA typing, it is impossible to establish unambiguously the origin of the Amerindian haplotype a in the family. Furthermore, only one of their children (individual no. 1) was HLA typed, in addition to several of their grandchildren and great-grandchildren. Given the validity of the family structure depicted in figure 1 (as stated earlier, we have no evidence to the contrary), the most likely interpretation is that PM carried the haplotype combination c/j and that his second wife, Maria Angata (MA), carried the haplotype combination a/e. Therefore, MA is the most probable ancestor of the Amerindian HLA haplotype a. Since MA was born on Easter Island in approximately 1846, this interpretation of the HLA data entails that the Amerindian haplotype a was introduced in Easter Island in good time prior to the Peruvian slave raids. Following this interpretation, individual 94 married to the granddaughter (no. 24; figure 1) of PM and MA also carried the Amerindian haplotype a. The results of the HLA investigations in this large family fully fit with these interpretations.
It should be added that there are alternative, but less likely interpretations of the available HLA data. One interpretation is that PM carried the Amerindian haplotype a (in addition to the European haplotype c). If PM carried haplotype a, then one would have to postulate an extramarital child in his large family. In any case, this would also place the introduction of the Amerindian haplotype a to Easter Island in very good time prior to the Peruvian slave raids. Another possibility is that neither PM nor MA carried the Amerindian haplotype a, but it was introduced in the family by the husbands of two of their daughters (no. 86 and their other daughter depicted to the far right in figure 1) as well as by individual no. 94. If so, the four investigated children of PM and MA must have inherited exactly the same HLA haplotypes from their parents (i.e. being c/e), which is quite unlikely (the chance is 1.5%). Furthermore, Langdon [20] states that all children of PM and MA married partners whose pure Easter Island ancestry may be traced back to the earliest church records. Thus, should this latter much more unlikely interpretation of the HLA data be true it would also strongly suggest an introduction of the Amerindian haplotype a on Easter Island in good time prior to the Peruvian slave raids.
The next question is how much earlier than the Peruvian slave trades in the 1860s the Amerindian HLA alleles may have been introduced on Easter Island. The following evidence suggests an early introduction:
— The Amerindian HLA alleles A*02:12 and B*39:05 were found on two different haplotypes (a and b) and both haplotypes are found in some unrelated individuals (figure 1 and discussed earlier). This suggests that the Amerindian alleles have been present on Easter Island for many generations.
— Haplotype b also carries the DRB1*04:03:01, DQA1*03 and DQB1*03:02 alleles which are more frequently found in Polynesia, as is also witnessed by their presence on the Polynesian haplotypes f and g. As discussed earlier, haplotype b may therefore be a recombinant Amerindian ACB/Polynesian DRDQ haplotype. Because the HLA genes are very closely linked on chromosome 6, recombinations in the HLA complex are rare and occur in approximately only 1 per cent of meioses. A recombinant Amerindian ACB/Polynesian DRDQ haplotype, therefore, also suggests that the Amerindian alleles must have been present on Easter Island for many generations.
— The Amerindian HLA alleles A*02:12 and B*39:05 occur together on the same haplotypes (a and b) on Easter Island. In Amerindians today, these alleles are more often found on separate haplotypes; i.e. not together. However, native Americans usually exhibit low allelic, but high haplotypic diversity [27]. Thus, the haplotypic combinations of HLA alleles in present day Amerindians may have diverged after the Amerindian HLA alleles reached Easter Island, also suggesting an early introduction on the island.
Exactly when these Amerindian HLA genes were introduced on Easter Island cannot be established by our studies. Our data provide strong evidence, however, that they were introduced prior to the Peruvian slave trades in the 1860s, and suggest that it occurred already in prehistoric time, but probably after the island was inhabited by Polynesians.
5. Investigations of new blood samples from Easter Island
To try to confirm our molecular genetic findings in the serum samples from 1971, new blood samples were collected in 2008 from 21 individuals who had not been included among those who gave their blood samples in 1971. They were carefully selected to be of Easter Island origin for many generations by information from several local persons with knowledge of the genealogy of early families of the island, and from written sources [28]. Some of the investigated individuals are related to some included in our previous investigations.
The new blood samples were investigated for genomic markers similar to the investigations of the 1971 samples, except that DNA was now extracted from a pellet of leucocytes. The results have been reported in detail elsewhere [29] and will be only briefly summarized here.
(a). Mitochondrial DNA data
All investigated individuals carried the full ‘Polynesian motif’. Two individuals had a ‘private’ substitution (C) at position 16126. No traces of mtDNA common in Amerindians were found, as was also the case in our investigations of the 1971 samples.
(b). Y chromosome data
The Y chromosome typing of the 11 males included in our investigations revealed that five of them had the C to T transition that characterizes haplogroup C-M208, abundant among Polynesian men. The others had Y chromosome markers possibly of European origin. Again, no traces of Y chromosome markers common in Amerindians were found.
(c). Genomic HLA data
Because the 21 investigated individuals were unrelated their HLA haplotypes had to be estimated, using methods described in Thorsby et al. [29]. Seven of the probable HLA haplotypes found were identical to some found in our previous studies, including haplotype c (see above). Eight HLA haplotypes not detected in our previous investigations were also found, six of which carried HLA alleles of probable Polynesian or European origin. Two haplotypes, however, carried some HLA alleles which most probably are Amerindian.
The latter two haplotypes are given in table 2. Haplotype 7, found in one individual, carried B*39:09 and DRB1*08:02, which have previously almost only been found among native South Americans [23,25]. The C*07:02 allele carried by this haplotype also has a very high frequency among native Americans, but is also found in some populations in southeast Asia and elsewhere [23,25]. Thus, the CBDR part of this haplotype is probably of Amerindian origin. Arnaiz-Villena et al. [26] also reported another haplotype, A*02-B*39-DRB1*09:01-DQ:B1*0303, which has also been found only in Aymara Amerindians living around Lake Titicaca. Using high-resolution retyping of this haplotype, it was found to carry the HLA alleles A*02:01-C*07:02-B*39:09-DRB1*09:01-DQB1*03:03 (Arnaiz-Villena, personal communication); i.e. the same ACB alleles as carried by Easter Island haplotype 7. Thus, Easter Island haplotype 7 may have been derived from this Aymara Amerindian haplotype by an ACB/DRDQ recombination.
Table 2.
Two HLA haplotypes with Amerindian alleles found in the samples collected in 2008.
| haplotypea | HLA-A* | HLA-C* | HLA-B* | HLA-DRB1* | HLA-DQA1* | probable origin |
|---|---|---|---|---|---|---|
| 7 | 02:01/02:09 | 07:02:01 | 39:09 | 08:02 | 04:01 | Amerindian (CBDR) |
| 8 | 24:02 | 03:03 | 15:07 | 04:04 | 03:01:01 | Amerindian (BDR) |
aHaplotype 7 was found in one individual, haplotype 8 in another.
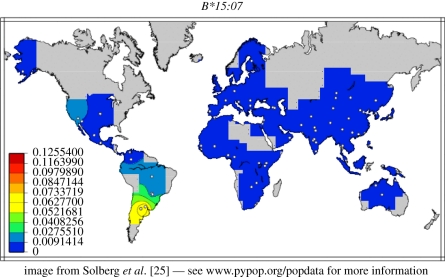
Haplotype 8, found in another individual, carried the HLA allele B*15:07, which is found in high frequency among natives in South America, in lower frequency among other Amerindians, but barely elsewhere (figure 3). The DRB1*04:04 allele on this haplotype also has a high frequency among native Americans, with lower frequencies in other populations [25]. Thus, the BDR part of this haplotype is probably also of Amerindian origin.
Figure 3.
The frequency of the HLA allele B*15:07 in various populations [25]. The allele frequencies are given in different colours to the left.
From the genealogy of the two individuals carrying haplotype 7 or 8, respectively, we cannot ascertain when these haplotypes were introduced on Easter Island. However, because both haplotypes may be the result of recombinations it is possible that they were introduced early and later recombined with other haplotypes on the island. It should also be noted that the Amerindian HLA alleles found in these recent investigations differ from those found in the 1971 samples (table 1), which indicates that an early Amerindian contribution to the gene pool of Easter Island might have been more than just trivial. In any case, the results of our more recent investigations support our findings in the 1971 samples.
6. No traces of Amerindians by studying mitochondrial DNA and Y chromosome markers
An early Amerindian contribution to the gene pool on Easter Island was revealed only by the genomic HLA investigations. No genetic traces of Amerindians were detected by typing for mtDNA and Y chromosome markers. One explanation for these contrasting findings is that the Amerindian HLA alleles may have been subject to different selective forces than Amerindian mtDNA and Y chromosome markers. The HLA alleles typed for encode molecules which are of great importance for immune responses, where different HLA molecules present different peptide fragments of antigens to T lymphocytes. Some Amerindian HLA alleles may have been selected which encode HLA molecules of importance for immune responses against given pathogens on Easter Island, whereas there was no similar selection of Amerindian mtDNA and Y chromosome markers.
There is, however, also another, not mutually exclusive explanation. At the end of the 1800s, approximately 100 individuals only were left on the island. Many had disappeared because of the Peruvian slave raids, others had died because of epidemics. This may cause a more random transmission of genes from one generation to the next; i.e. genetic drift, leading to random genetic changes in the population including loss of given gene variants. Uniparental genetic markers such as mtDNA and Y chromosome markers are more sensitive to genetic drift than genes which are inherited from both parents, such as the HLA genes. This also illustrates the usefulness of genomic HLA typing to complement mtDNA and Y chromosome analyses in anthropological investigations.
7. How did the early Amerindians reach Easter Island?
First, it should be noted that we cannot completely exclude that the source of these early Amerindian genes may be Amerindian crewmembers on some ships visiting Easter Island before the mid 1800s. We lack information, however, that Amerindians were among the crew of any these early visiting ships.
If some Amerindians reached Easter Island in prehistoric time, as our investigations suggest, how did they arrive? The answer to this question cannot of course be answered by our investigations. However, there are at least two possible, not mutually exclusive, explanations.
One possibility is that some native Americans arrived at Easter Island on balsa rafts, possibly via other Polynesian islands, by following westbound ocean currents from the northern parts of South America. By his Kon-Tiki expedition in 1947, Heyerdahl showed that it was possible to reach Polynesia on a balsa raft from Peru.
Another possibility is that some Polynesians sailed further east in their canoes and reached South America [6]. There is no doubt that they were good sailors. If they followed a more southern route in the Pacific, then they would be helped by more eastbound ocean currents and westerly winds. After we had finished our molecular genetic studies of the 1971 serum samples, Storey et al. [30,31] analysed the remains of some chickens found in El Arenal in the southern part of Chile. Most interestingly, by mtDNA analysis they found that the chickens were of Polynesian type, while radiocarbon investigations showed that the remains dated back to 1300–1400. Furthermore, suggestive evidence of skeletal remains of pre-Columbian Polynesian ancestry at the Mocha Island, Chile was also recently reported [32]. Thus, there is strong evidence that Polynesians had been in South America early, i.e. in pre-Columbian time (see also Lawler [33]). After having arrived in South America, some of them may have returned to Polynesia, including Easter Island, not only taking the sweet potato and bottle gourd, etc., but also some native Americans with them.
8. Conclusions
The results of our molecular genetic studies of some highly selected Easter Islanders are fully compatible with the notion that the first inhabitants of the island were Polynesians arriving from the west. Our investigations of HLA alleles in the studied population, however, also demonstrate for the first time early genetic traces of some Amerindians on the island.
We cannot establish by our investigations when the first Amerindians reached Easter Island. The data provide strong evidence, however, for their introduction prior to the Peruvian slave trades in the 1860s, and suggest that it occurred in prehistoric time, but probably after the island was inhabited by Polynesians.
Further studies on HLA and other genetic markers from DNA collected from prehistoric skeletal material from Easter Island and other Polynesian islands are necessary to provide more exact information on when the first Amerindians may have arrived in the area. The very recent report on putative HLA alleles of some archaic individuals (Neanderthals, Denisovans), obtained by reanalysing genomic sequence data collected from bones [34], suggests that this may be possible in the near future.
Acknowledgements
This paper is a revised, extended and updated version of a previous review [35] and summarizes the work of many. Their names can be found from the list of authors of and acknowledgements in references [17,22,29], and are all gratefully acknowledged for a most fruitful collaboration for many years. I also thank Antonio Arnaiz-Villena, Department of Immunology, University Complutense, Madrid, Spain for high-resolution typing of some Aymara Amerindian haplotypes. Many thanks to Paul Wallin, Department of Archaeology and Osteology, University of Gotland, Sweden and to Erika Hagelberg, Department of Biology, University of Oslo, Norway for helpful comments on the manuscript. Major financial support was received from Oslo University Hospital, Rikshospitalet and Medinnova, Rikshospitalet.
ENDNOTE
For a short description of the very polymorphic HLA genetic complex, the nomenclature used for its alleles and its informative value as a tool in anthropological investigations, see Sanchez-Mazas et al. [23].
References
- 1.Friedlander J. S., et al. 2008. The genetic structure of Pacific islanders. PLoS Genet. 4, 0173–0190 10.1371/journal.pgen.0040019 (doi:10.1371/journal.pgen.0040019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagelberg E., Clegg J. B. 1993. Genetic polymorphisms in prehistoric Pacific islanders determined by analysis of ancient bone DNA. Proc. R. Soc. Lond. B 252, 163–170 10.1098/rspb.1993.0061 (doi:10.1098/rspb.1993.0061) [DOI] [PubMed] [Google Scholar]
- 3.Sykes B., Leiboff A., Low-Bear J., Tetzner S., Richards M. 1995. The origins of the Polynesians: an interpretation from mitochondrial lineage analysis. Am. J. Hum. Genet. 57, 1463–1475 [PMC free article] [PubMed] [Google Scholar]
- 4.Kayser M. 2010. The human genetic history of Oceania: near and remote views of dispersal. Curr. Biol. 20, R194–R201 10.1016/j.cub.2009.12.004 (doi:10.1016/j.cub.2009.12.004) [DOI] [PubMed] [Google Scholar]
- 5.Kayser M., Brauer S., Weiss G., Underhill P. A., Roewer L., Schiefenhovel W., Stoneking M. 2000. Melanesian origin of Polynesian Y chromosomes. Curr. Biol. 10, 1237–1246 10.1016/S0960-9822(00)00734-X (doi:10.1016/S0960-9822(00)00734-X) [DOI] [PubMed] [Google Scholar]
- 6.Martinsson-Wallin H., Crockford S. J. 2002. Early settlement of Rapa Nui (Easter Island). Asia Perspect. 40, 244–278 10.1353/asi.2001.0016 (doi:10.1353/asi.2001.0016) [DOI] [Google Scholar]
- 7.Hunt T. L., Lipo C. P. 2006. Late colonization of Easter Island. Science 311, 1603–1606 10.1126/science.1121879 (doi:10.1126/science.1121879) [DOI] [PubMed] [Google Scholar]
- 8.Yen D. E. 1974. The sweet potato and Oceania. Bulletin no. 236, Bernice P. Bishop Museum, Honolulu, Hawaii
- 9.Wallin P., Stevenson C., Ladefoged T. 2005. Sweet potato production on Rapa Nui. In Ethnology monographs 19 (eds Ballard C., Brown P., Bourke R. M., Harwood T.), Oceania Monograph 56. [Google Scholar]
- 10.Green R. C. 2000. A range of disciplines support a dual origin for the bottle gourd in the Pacific. J. Polynesian Soc. 109, 191–197 [Google Scholar]
- 11.Martinez A. L. 1979. 9700 years of maritime subsistence on the Pacific: an analysis by means of bioindicators in the north of Chile. Am. Antiq. 44, 309–324 10.2307/279082 (doi:10.2307/279082) [DOI] [Google Scholar]
- 12.Klar K. A. 2010. Linguistic evidence for prehistoric Polynesian–American contact. In Migration, identity and cultural heredity. Selected papers from the 7th Int. Conf. on Easter Island and the Pacific (eds Wallin P., Martinsson-Wallin H.), pp. 319–324 Visby, Sweden: Gotland University Press [Google Scholar]
- 13.Jones T. L. 2010. Polynesians in the new world: the Chumash connection and beyond. In Migration, identity and cultural heredity. Selected papers from the 7th Int. Conf. on Easter Island and the Pacific (eds Wallin P., Martinsson-Wallin H.), pp. 307–318 Visby, Sweden: Gotland University Press [Google Scholar]
- 14.Heyerdahl T. 1952. American Indians in the Pacific. The theory behind the Kon-Tiki expedition. London, UK: Allen and Unwin [Google Scholar]
- 15.Bonatto S. L., Redd A. J., Salzano F. M., Stoneking M. 1996. Lack of ancient Polynesian–Amerindian contact. Am. J. Hum. Genet. 59, 253–256 [PMC free article] [PubMed] [Google Scholar]
- 16.Hurles M. E., Maund E., Nicholson J., Bosch E., Renfrew C., Sykes B. C., Jobling M. A. 2003. Native American Y chromosomes in Polynesia: the genetic impact of the Polynesian slave trade. Am. J. Hum. Genet. 72, 1282–1287 10.1086/374827 (doi:10.1086/374827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorsby E., Colombani J., Dausset J., Figueroa F., Thorsby A. 1973. HLA, blood group and serum type polymorphism of natives on Easter Island. In Histocompatibility testing 1972 (eds Dausset J., Colombani J.), pp. 287–302 Copenhagen, Denmark: Munksgaard [Google Scholar]
- 18.Hagelberg E., Quevedo S., Turbon P., Clegg J. B. 1994. DNA from ancient Easter Islanders. Nature 369, 25–26 10.1038/369025a0 (doi:10.1038/369025a0) [DOI] [PubMed] [Google Scholar]
- 19.González-Pérez E., Esteban E., Via M., García-Maro C., Hernández-Moro M., Moral P. 2006. Genetic change in the Polynesian population of Easter Island: evidence from Alu insertion polymorphisms. Ann. Hum. Genet. 70, 829–840 10.1111/j.1469-1809.2006.00293.x (doi:10.1111/j.1469-1809.2006.00293.x) [DOI] [PubMed] [Google Scholar]
- 20.Langdon R. 1988. The lost caravel re-explored. Canberra, Australia: Brolga Press [Google Scholar]
- 21.McCall G. 1976. Reaction to disaster: continuity and change in Rapanui social organisation, p. 316 PhD thesis, Australian National University, Canberra [Google Scholar]
- 22.Lie B. A., Dupuy B. M., Spurkland A., Fernandez-Vina M. A., Hagelberg E., Thorsby E. 2007. Molecular genetic studies of natives on Easter Island: evidence of an early European and Amerindian contribution to the Polynesian gene pool. Tissue Antigens 69, 10–18 10.1111/j.1399-0039.2006.00717.x (doi:10.1111/j.1399-0039.2006.00717.x) [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Mazas A., et al. 2011. Immunogenetics as a tool in anthropological studies. Immunology 133, 143–164 10.1111/j.1365-2567.2011.03438.x (doi:10.1111/j.1365-2567.2011.03438.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velickovic Z. M., Delahunt B., Carter J. M. 2002. HLA-DRB1 and HLA-DQB1 polymorphisms in Pacific Islands populations. Tissue Antigens 59, 397–406 10.1034/j.1399-0039.2002.590506.x (doi:10.1034/j.1399-0039.2002.590506.x) [DOI] [PubMed] [Google Scholar]
- 25.Solberg O. D., Mack S. J., Lancaster A. K., Single R. M., Tsai Y., Sanchez-Mazas A., Thomson G. 2008. Balancing selection and heterogeneity across the classical human leukocyte antigen loci: a meta-analytic review of 497 population studies. Hum. Immunol. 69, 443–464 10.1016/j.humimm.2008.05.001 (doi:10.1016/j.humimm.2008.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnaiz-Villena A., Parga-Lozano C., Moreno E., Areces C., Rey D., Gomez-Prieto P. 2010. The origin of Amerindians and peopling of the Americas according to HLA genes: admixture with Asian and Pacific people. Curr. Genomics 11, 103–114 10.2174/138920210790886862 (doi:10.2174/138920210790886862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollenbach J. A., Thomson G., Cao K., Fernandez-Vina M., Erlich H. A., Bugawan T. L., Winkler C., Winter M., Klitz W. 2001. HLA diversity, differentiation, and haplotype evolution in mesoAmerican natives. Hum. Immunol. 62, 378–390 10.1016/S0198-8859(01)00212-9 (doi:10.1016/S0198-8859(01)00212-9) [DOI] [PubMed] [Google Scholar]
- 28.Alberto Hotus y otros; El Consejo de Jefes de Rapa Nui 1988. Te Mau Hatu'O Rapa Nui. Rapa Nui: Centro de Estudios Politicos Latinoamericanos Simon Bolivar
- 29.Thorsby E., Flåm S. T., Woldseth B., Dupuy B. M., Sanches-Mazas A., Fernandez-Vina M. A. 2009. Further evidence of an Amerindian contribution to the Polynesian gene pool on Easter Island. Tissue Antigens 73, 582–585 10.1111/j.1399-0039.2009.01233.x (doi:10.1111/j.1399-0039.2009.01233.x) [DOI] [PubMed] [Google Scholar]
- 30.Storey A. A., et al. 2007. Radiocarbon and DNA evidence for a pre-Columbian introduction of Polynesian chickens to Chile. Proc. Natl Acad. Sci. USA 104, 10 335–10 339 10.1073/pnas.0703993104 (doi:10.1073/pnas.0703993104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storey A. A., et al. 2008. Pre-Columbian chickens, dates, isotopes, and mtDNA. Proc. Natl Acad. Sci. USA 105, E99. 10.1073/pnas.0807625105 (doi:10.1073/pnas.0807625105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matisoo-Smith E., Ramirez J. M. 2010. Human skeletal evidence of Polynesian presence in South America? Metric analyses of six crania from Mocha Island, Chile. J. Pac. Arch. 1, 76–88 [Google Scholar]
- 33.Lawler A. 2010. Beyond Kon-Tiki: did Polynesians sail to South America? Science 328, 1344–1347 10.1126/science.328.5984.1344 (doi:10.1126/science.328.5984.1344) [DOI] [PubMed] [Google Scholar]
- 34.Abi-Rached L., et al. 2011. The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 334, 89–94 10.1126/science.1209202 (doi:10.1126/science.1209202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorsby E. 2010. Evidence of an early Amerindian contribution to the Polynesian gene pool on Easter Island. In Migration, identity, and cultural heredity. Selected Papers from the 7th Int. Conf. on Easter Island and the Pacific (eds Wallin P., Martinsson-Wallin H.), pp. 285–295 Visby, Sweden: Gotland University Press [Google Scholar]