Table 3.
NHC–Cu-Catalyzed Allenyl Additions to Aryl-Containing Disubstituted Allylic Phosphatesa
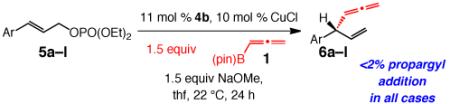
| entry | Ar | conv (%);b yield (%)c |
SN2′:SN2b | erd |
|---|---|---|---|---|
| 1 | Ph; 5a | >98; 79 | 96:4 | 95.5:4.5 |
| 2 | o-FC6H4, 5b | >98; 79 | 94:6 | 96:4 |
| 3 | o-BrC6H4, 5c | >98; 80 | 88:12 | 96:4 |
| 4 | o-CF3C6H4, 5d | >98; 67 | 77:23 | 96:4 |
| 5 | o-MeC6H4, 5e | >98; 68 | >98:2 | 95:5 |
| 6 | o-MeOC6H4, 5f | >98; 79 | >98:2 | 96.5:3.5 |
| 7 | m-BrC6H4, 5g | >98; 92 | 97:3 | 98.5:1.5 |
| 8 | m-CF3C6H4, 5h | >98; 65 | 91:9 | 98:2 |
| 9 | m-MeC6H4, 5i | >98; 71 | >98:2 | 97:3 |
| 10 | 2-naphthyl, 5j | >98; 88 | 98:2 | 99:1 |
| 11 | p-ClC6H4, 5k | >98; 64 | 93:7 | 96:4 |
| 12 | p-O2NC6H4, 5l | >98; 69 | 85:15 | 97 :3 |
Reactions performed under N2 atm.
Determined by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; conversion refers to consumption of the substrate.
Yields of isolated and purified allenyl addition products.
Determined by GLC analysis; see the Supporting Information for details.
