Abstract
The development of factor VIII inhibitors in non-hemophilic patients is rare and may occur in healthy individuals, mostly elderly and women in postpartum period, and in patients with malignant neoplasia or autoimmune diseases, such as bullous pemphigoid. We described the case of a 60-year-old female patient who developed bullous pemphigoid for 3 month and presented with bleeding tendency and hematoma in the tongue. Therapy with methylprednisolone, cyclophosphamide, intravenous immunoglobulin and factor VIII reposition was instituted, resulting in a remission of the bleeding and negativity for antibodies against factor VIII titers. We concluded that, despite its rarity, the presence of acquired factor VIII inhibitors should be investigated when patients with autoimmune diseases develop bleeding manifestations.
Keywords: Bullous pemphigoid, factor VIII inhibitor, acquired hemophilia
Introduction
Acquired hemophilia (AH) is a rare disorder that can lead to severe and life-threatening bleeding, usually caused by spontaneous development of autoantibody against factor VIII [1]. In the majority of cases, their presence is idiopathic but they can be associated with pregnancy, post-partum period, malignancy, drug reaction or autoimmune diseases[2]. In bullous pemphigoid (BP), an association with AH has been only rarely described, in which case the prognosis is consistently very poor. The mortality has been estimated at 22% [3]. 11 cases of BP associated with AH have been published [4]. In this report, we describe a patient with BP who developed acquired hemophilia.
Case Report
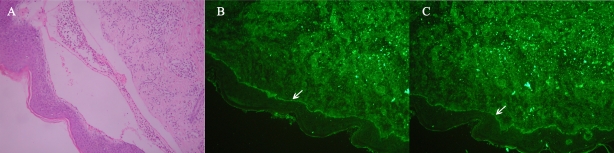
We report the case of a 60-yr-old Chinese woman who complained with a 3-month history of scattered blister and hemorrhagic bullae located on extremities and trunk which led to the diagnosis of bullous pemphigoid. To confirm the diagnosis of BP, skin biopsy was performed. Preoperative clotting test shown a slight prolonged activated partial thromboplastin time (APTT) (59s, N=30∼44s) with a normal prothrombin time (13.8s) and thrombin time (15.2 s). On the third postoperative day (POD) the patient complained of bleeding in biopsy site and a size of pigeon egg hematoma in base of tongue was presented. The bleeding was persisted for one week despite suture, a progressively more severe anemia (hemoglobin 6.9 mg/ dl on POD 5) were noticed. Clotting tests showed an extremely long APTT (100.6 second). Coagulation factor assay (factors VIII, IX, XI, XII) revealed a low level of factor VIII (10.5%, N = 60-150%) and the presence of factor VIII inhibitor. The results of histopathology and direct immunofluorescence confirmed the diagnosis of bullous pemphigoid. Skin biopsy revealed a subepidermal blister present with a dermal mononuclear and eosinophils infiltrate (Figure 1A). Direct immunofluorescence (DIF) showed linear deposition of C3 and IgG along the dermoepidermal junction (Figure 1B and 1C). In addition, IgG autoantibody targeted BP180 is positive (47.2U/ml, threshold 9 U/ml), desmoglein 1 (DSG1) and desmoglein 3 (DSG3) is negative. Anti-Sm and antinuclear antibody (ANA) were positive. The results of the other tests such as blood platelet number, rheumatoid factor, anticardiolipin IgG, and HIV were all normal or negative.
Figure 1.
(A) Lesional skin biopsy specimens reveal a subepidermal blister, Perivascular and interstitial infiltrate of lymphocytes and eosinophils in the upper part of the reticular dermis. (B) Direct immunofluorescence study performed on skin biopsy specimen shows a linear band of immunoglobulin G deposit along the dermoepidermal junction. (C) Direct immunofluorescence study performed on skin biopsy specimen shows a linear band of C3 deposit along the dermoepidermal junction.
Her past medical history revealed mild hypertension, gallstone disease and uterine fibroids. Her hypertension was treated with metoprolol and nifedipine. She had undergone myomectomy 7 year ago without complications. The absence of familial and personal bleeding history plus laboratory tests, accompanied by the fact that the APTT was not corrected following the infusion of normal plasma led us to presume that a coagulation inhibitor was involved. A diagnosis of AH associated with BP was made. After consultation with senior hematologists, we decided to transfuse packed red blood cell, fresh frozen plasma and recombinant human factor VIII. Systemic corticosteroids (methylprednisolone 40mg/d) in combination with cyclophosphamide (800 mg i.v. once a month for 3 months) and intravenous immunoglobulin (20g/d for 5 days) were subsequently administered. Her APTT gradually normalized and bleeding was controlled at POD 14. After a month, we noted fewer than 10 bullae, APTT normalized (26 seconds), the factor VIII level climbed back up to 125%, and we could no longer detect anti-factor VIII inhibitor. The coagulation profile was normal. 10 months after the first onset of AH, the patient was followed up with complete remission. She reported no spontaneous bleeding episodes. She remained on tapering doses of corticosteroids (prednisone 15mg/d) as treatment for BP 18 months after the first onset of AH.
Discussion
Bullous pemphigoid is a chronic, autoimmune, subepidermal, blistering skin disease that rarely involves mucous membranes. Bullous pemphigoid is characterized by the presence of immunoglobulin G (IgG) autoantibodies specific for the hemidesmosomal bullous pemphigoid antigens BP230 (BPAg1) and BP180 (BPAg2). The association of BP with some other diseases, such as psoriasis vulgaris and vitiligo, squamous cell carcinoma, has been mentioned before[5.6]. Autoantibodies against factor VIII are an extremely rare complication during BP. The etiology of AH remains unclear. There are several hypotheses proposed to explain the relationship between BP and AH[4]. The serum anti-factor VIII antibody may interact with the central collagen-like part of the BPAG2 protein because there is sequence homology between epitopes on factor VIII and the BPAG2 collagen XVII domain. AH should be suspected in patients who have never had any signs of bleeding tendency earlier. In contrast to classical hemophilia, spontaneous bleeds to joints are rarely observed in the course of AH. Easy bruising with extensive enlargement, muscle hematomas and profuse bleeds after trauma and surgery are the most common symptoms [7]. Extraordinary bleeds may also occur elsewhere as in the gastrointestinal tract, the retroperitoneal space. Severe bleeds may be life-threatening. The typical findings of acquired hemophilia are that the activated partial thromboplastin time (APTT) is prolonged and factor VIII level is decreased but the thrombin times, prothrombin times, the platelet count and function are normal[8]. Early diagnosis is extremely important, because, although it is a rare condition, it is also extremely severe and has high mortality.
Treatment should be focused on controlling the immediate bleeding episode and suppressing the immune reaction against the coagulant factor. Immunosuppressive agents can be used, such as corticosteroids, cyclophosphamide, azathioprine, ciclosporin, or high dose immunoglobulin [7]. It has become clear that rituximab may be a valuable agent in managing acquired hemophilia [7.8]. Oral corticosteroid drugs also are the primary treatment for BP. Severe bullous pemphigoid can also require immune-suppression drugs such as azathioprine, cyclophosphamide. This case report shows a female patient with autoimmune disease who has developed acquired hemophilia and has been successfully treated with a combination of fresh frozen plasma, human factor VIII, methylprednisolone, cyclophosphamide, and intravenous immunoglobulin. Our patient responded well to immunosuppressive therapy.
In summary, AH is caused by autoantibodies (so-called inhibitors) to coagulation factors (mostly Factor VIII). Patient can be often identified by a history of unexplained bleeding episodes and by a prolonged APTT. Because bleeding is often severe, a prompt and accurate diagnosis of bullous pemphigoid associated with acquired hemophilia is necessary in order to provide adequate therapy.
References
- 1.Kessler CM, Asatiani E. In: Acquired Inhibitors to Factor VIII. Textbook of Hemophilia. Lee CA, Berntorp EE, Hoots WK, Aledort LM, editors. Malden: Blackwell Publishing Ltd; 2007. pp. 86–90. [Google Scholar]
- 2.Franchini M, Lippi G. Acquired factor VIII inhibitors. Blood. 2008;112:250–5. doi: 10.1182/blood-2008-03-143586. [DOI] [PubMed] [Google Scholar]
- 3.Green D, Lechner K. A survey of 215 non-hemophilic patients with inhibitors to factor VIII. Thromb Haemost. 1981;45:200–3. [PubMed] [Google Scholar]
- 4.Caudron A, Chatelain D, Christophe O, Lok C, Roussel B, Viseux V. Favourable progression of acquired hemophilia-associated bullous pemphigoid. Eur J Dermatol. 2009;19:383–4. doi: 10.1684/ejd.2009.0672. [DOI] [PubMed] [Google Scholar]
- 5.Pasic A, Ljubojevic S, Lipozencic J, Marinovic B, Loncaric D. Coexistence of psoriasis vulgaris, bullous pemphigoid and vitiligo: a case report. J Eur Acad Dermatol Venereol. 2002;16:426–7. doi: 10.1046/j.1468-3083.2002.00570_12.x. [DOI] [PubMed] [Google Scholar]
- 6.Deguchi M, Tsunoda T, Tagami H. Resolution of bullous pemphigoid and improvement of vitiligo after successful treatment of squamous cell carcinoma of the skin. Clin Exp Dermatol. 1999;24:14–5. doi: 10.1046/j.1365-2230.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- 7.Tengborn L, Astermark J, Ingerslev J, Mäkipernaa A, Tjønnfjord GE, Önundarson PT. Acquired Hemophilia-Guidelines. 2009. http://nordhemophilia.org/newpage.php?page=link5&idsublink=32.
- 8.Giangrande P. Acquired Hemophilia. 2005. http://www.wfh.org/2/docs/Publications/Diagnosis_and_Treatment/TOH38_Acquired_Hemophilia.pdf.