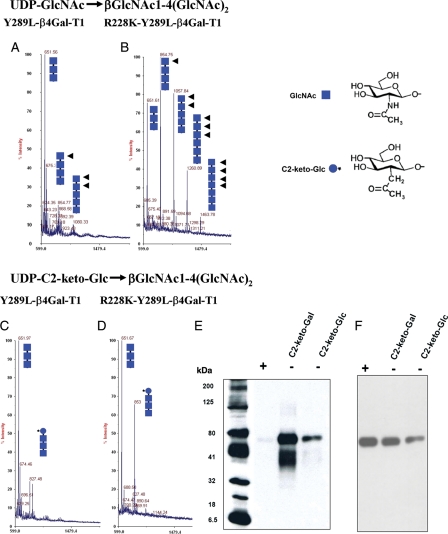
Fig. 2.
MALDI mass spectra of GlcNAc-T catalytic reaction using the acceptor chitotriose [(GlcNAcβ1,4-(GlcNAcβ1,4)2], by the enzymes Y289L-β4Gal-T1 (A) and R228K-Y289L-β4Gal-T1 (B). The chitotriose in both the reactions is elongated by the enzymes, because in the GlcNAc-T reaction, the product with GlcNAc at the non-reducing end (squares with arrows) is an acceptor substrate for the enzyme; thus, the initial acceptor substrate is elongated by these enzymes. Under similar reaction conditions and enzyme concentrations, the double mutant is better than the single-mutant enzyme in the GlcNAc-T catalytic reaction. The MALDI mass spectra of the catalytic reaction, using the same acceptor substrate chitotriose with UDP-C2-keto-Glc as the donor substrate, by the mutant enzymes Y289L-β4Gal-T1and R228K-Y289L-β4Gal-T1 are shown in (C) and (D), respectively. The double mutant shows a major peak at m/z 853, which corresponds to the product after the transfer of the C2-keto-Glc (blue circle with star) to the acceptor sugar chitotriose, which is absent in the transfer with the single mutant. However, no elongation of the acceptor substrate is seen with C2-keto-Glc, indicating that C2-keto-Glc, though it is an isostere of GlcNAc, is not an acceptor sugar for these enzymes. (E) The chemoenzymatic detection of C2-keto-Glc or C2-keto-Gal transferred to Ova. The transfer of C2-keto-Glc or C2-keto-Gal to GlcNAc residues on the N-glycans chains of Ova by the R228K-Y289L-β4Gal-T1 was monitored by linking the product with AOB, followed by western blotting and chemiluminescence detection. Chemiluminescence was detected only in the samples that contained C2-keto-Glc or C2-keto-Gal enzyme and 25 ng of Ova. After the transfer of C2-keto-Glc or C2-keto-Gal, a portion of Ova samples was treated with PNGase F, which removes the N-glycan chains from the protein (+). In contrast to the PNGase F-treated samples (+), the untreated samples (−) exhibited chemiluminescence, indicating that C2-keto-Glc or C2-keto-Gal is selectively transferred to the glycan portion of the Ova molecule. (F) The amount of Ova samples loaded in each lane was monitored by treating the membranes with the monoclonal antibody against Ova after western blot analysis.