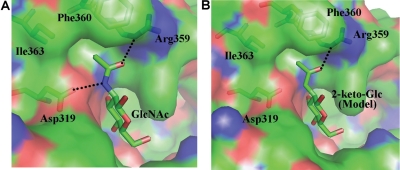
Fig. 6.
(A) The binding of GlcNAc molecule to β4Gal-T1 enzyme. The β4Gal-T1 enzyme is shown in a molecular surface diagram; the GlcNAc molecule and the protein residues that are interacting with its N-acetyl moiety are shown in a stick diagram (PDB 1NQI). The hydrogen bonds are shown in dotted black lines. (B) Based on the GlcNAc binding to β4Gal-T1 interactions, the 2-keto-Glc binding has been modeled without any energy minimization. Due to the absence of the amino group in the C2-keto-Glc molecule, the hydrogen bond between the amino group and the side-chain carboxylate oxygen atom of Asp319 residue is absent here.