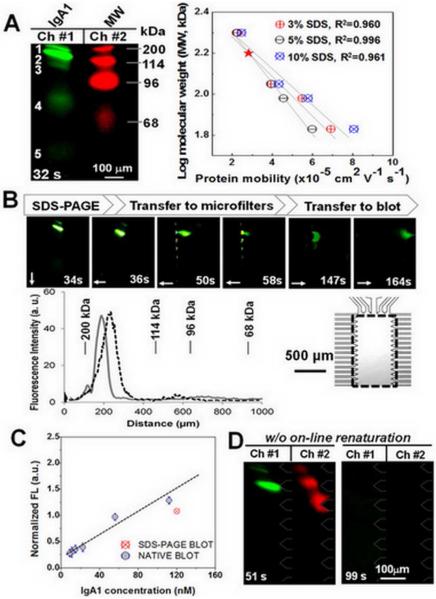
Figure 4.
Microfluidic HAA lectin blot of galactose-deficient IgA1 myeloma protein. (A) Fluorescence micrographs show two-color monitoring of MW ladders and myeloma IgA1 sizing. The linear calibration curves (right) were obtained using varied concentrations of SDS for calculating unknown protein MW (myosin heavy chain 200 kDa, β-galactosidase 114 kDa, phosphorylase B 96 kDa, and human serum albumin 68 kDa). The red star indicates the size of monomeric IgA1. (B) Fluorescence micrographs report time evolution of HAA lectin blot of galactose-deficient IgA1 myeloma protein. Plot of fluorescence intensity distribution on separation axis (gray line) is compared to intensity distribution in blotting array (dashed black line at 164 s). Arrows indicate the direction of electrophoresis. Array channel spacing is ~50 um. Imaging region is shown in inset. (C) Evaluation of the recovered activity by comparison of captured myeloma IgA1 amount in blotting region under native and SDS conditions. (D) HAA blot of 5% SDS-treated myeloma IgA1 (green) and MW ladders (68-200 kDa, red) without on-line renaturation, as negative control.