Figure 3.
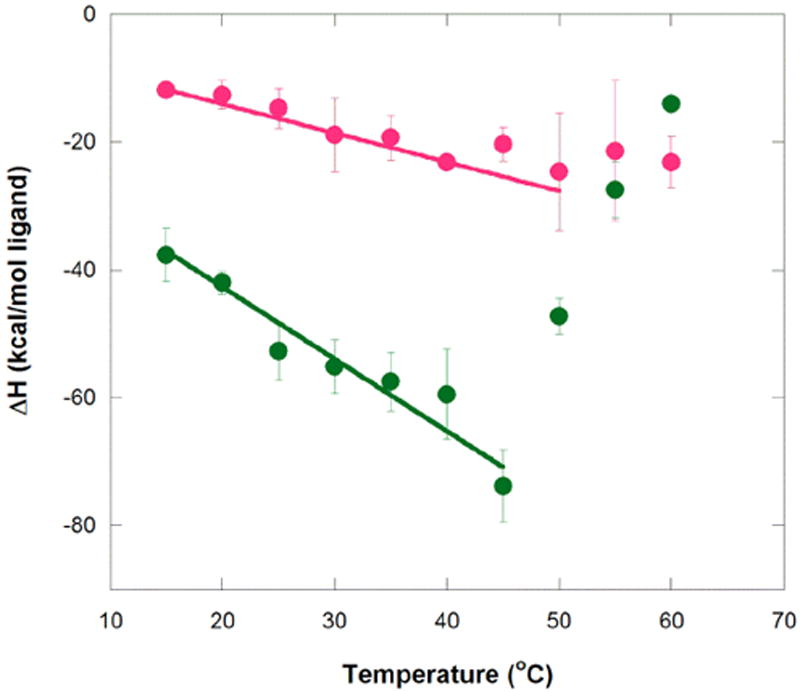
Temperature dependence of enthalpy for aptamer binding to MG and TMR. Circles represent average ΔH° values at a given temperature for MG (green) and TMR (magenta). Error bars are the standard error of the mean. Weighted linear regression of MG binding over the temperature range of 15 to 45 °C gives ΔH(T) = −1.13 kcal/mol°C (T − 25 °C) − 48.3 kcal/mol (R2 = 0.95), while that for TMR binding over the range 15 to 50 °C gives ΔH(T) = −0.45 kcal/mol°C (T − 25 °C) − 16.3 kcal/mol (R2 = 0.97). These plots provide ΔCp values for MG and TMR of −1.13+/−0.17 and −0.45+/−0.035 kcal K−1 mol−1, respectively. Note that the values of the final term in these fits are similar to the actual enthalpies for MG and TMR measured at 25 °C (provided in Tables 1 and 2), as expected. Buffer conditions were 10 mM sodium cacodylate (pH 5.8), 10 mM KCl, and 10 mM MgCl2.
