The correlation between quantitative human epidermal growth factor receptor (HER)-2 protein expression in primary breast cancers and the time to brain metastases in HER-2+ advanced breast cancer patients treated with trastuzumab was investigated. A strong relationship between the quantitative HER-2 protein expression level and the risk for brain relapse in HER-2+ advanced breast cancer patients was found.
Keywords: Advanced breast cancer, Brain metastasis, HER-2 amplification, Quantitative HER-2 protein level, Trastuzumab
Abstract
Background.
Patients with human epidermal growth factor receptor (HER)-2+ breast cancer are at particularly high risk for brain metastases; however, the biological basis is not fully understood. Using a novel HER-2 assay, we investigated the correlation between quantitative HER-2 expression in primary breast cancers and the time to brain metastasis (TTBM) in HER-2+ advanced breast cancer patients treated with trastuzumab.
Methods.
The study group included 142 consecutive patients who were administered trastuzumab-based therapy for HER-2+ metastatic breast cancer. HER-2/neu gene copy number was quantified as the HER-2/centromeric probe for chromosome 17 (CEP17) ratio by central laboratory fluorescence in situ hybridization (FISH). HER-2 protein was quantified as total HER-2 protein expression (H2T) by the HERmark® assay (Monogram Biosciences, Inc., South San Francisco, CA) in formalin-fixed, paraffin-embedded tumor samples. HER-2 variables were correlated with clinical features and TTBM was measured from the initiation of trastuzumab-containing therapy.
Results.
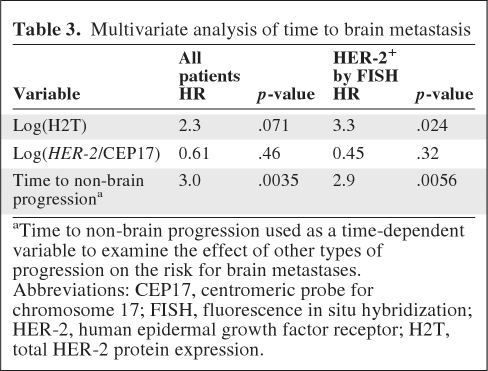
A higher H2T level (continuous variable) was correlated with shorter TTBM, whereas HER-2 amplification by FISH and a continuous HER-2/CEP17 ratio were not predictive (p = .013, .28, and .25, respectively). In the subset of patients that was centrally determined by FISH to be HER-2+, an above-the-median H2T level was significantly associated with a shorter TTBM (hazard ratio, [HR], 2.4; p = .005), whereas this was not true for the median HER-2/CEP17 ratio by FISH (p = .4). Correlation between a continuous H2T level and TTBM was confirmed on multivariate analysis (HR, 3.3; p = .024).
Conclusions.
These data reveal a strong relationship between the quantitative HER-2 protein expression level and the risk for brain relapse in HER-2+ advanced breast cancer patients. Consequently, quantitative assessment of HER-2 protein expression may inform and facilitate refinements in therapeutic treatment strategies for selected subpopulations of patients in this group.
Introduction
Breast cancer is a malignancy with a remarkably high risk for brain relapse [1, 2]. Brain metastases accompanying breast cancer are associated with a poor prognosis, negatively impact quality of life, and are relatively resistant to systemic therapies. A particularly high risk for brain relapse is associated with overexpression of human epidermal growth factor receptor (HER)-2 or amplification of the HER-2/neu gene [3–5], which is most likely related to the molecular characteristics of this tumor type and to the poor efficacy of systemic therapy to prevent brain metastases [6]. Growing evidence suggests that the high incidence of brain metastasis with breast cancer may be attributed to organ-specific tropism [7, 8]. Trastuzumab, a monoclonal antibody that targets the extracellular domain of HER-2, is a standard component of systemic therapy for HER-2+ breast cancer. Although, on average, trastuzumab treatment is associated with considerable benefits in terms of progression-free and overall survival (OS) outcomes, only a fraction of HER-2+ metastatic breast cancer patients respond to this agent, and a significant proportion of responders relapse within 1 year [9–13]. Importantly, because of its high molecular weight (145,000 Da) and other physical and chemical properties, trastuzumab does not cross an intact blood–brain barrier and is ineffective in preventing and treating brain metastases [14, 15]. Consequently, 30%–50% of HER-2+ advanced breast cancer patients develop brain relapse [16–24], with an annual risk of ∼10% [24]. As a result of the impaired penetration of trastuzumab across the blood–brain barrier, brain metastases frequently occur in patients with responsive or stable disease at metastatic extracranial sites [25, 26]. On the other hand, better control of extracranial metastatic disease resulting from trastuzumab therapy was found to delay the development of brain relapse [27, 28], and the continuation of trastuzumab beyond brain progression results in a longer survival time [23, 28–30].
Several retrospective studies have explored clinical and biological features associated with a propensity to develop brain relapse in patients with HER-2+ advanced breast cancer. Reported adverse factors include the presence of visceral disease, younger age, premenopausal status, a short disease-free interval after primary therapy, and a negative hormone receptor status [16–19, 22–24]. However, the results of particular studies have been inconsistent and none of these factors alone or in combination could enable selection of a subset of HER-2+ advanced breast cancer patients who might benefit from active surveillance for brain relapse or from potential preventive strategies. Recently, expression of several genes was found to be associated with a higher risk for brain relapse in both the general population of breast cancer patients [7] and the HER-2+ subset [31]; however, no robust molecular signature to predict brain relapse has been developed.
The VeraTagTM proximity-based assay (HERmark® Breast Cancer Assay; Monogram Biosciences, Inc., South San Francisco, CA) enables precise quantitative measurements of total HER-2 expression in formalin-fixed, paraffin-embedded tissue specimens [32]. Most recently, higher HER-2 expression, as determined using this assay, was associated with a longer survival time after trastuzumab treatment in HER-2+ advanced breast cancer patients [33, 34]; however, the association between the quantitative HER-2 level (H2T) and the propensity to metastasize to a particular site has not been examined. This study was designed to investigate the correlation between a continuous HER-2 level as measured by the HERmark Assay and the risk for brain relapse in HER-2+ advanced breast cancer patients treated with trastuzumab.
Materials and Methods
This study was approved by the Ethics Committee of the Medical University in Gdańsk, the coordinating center.
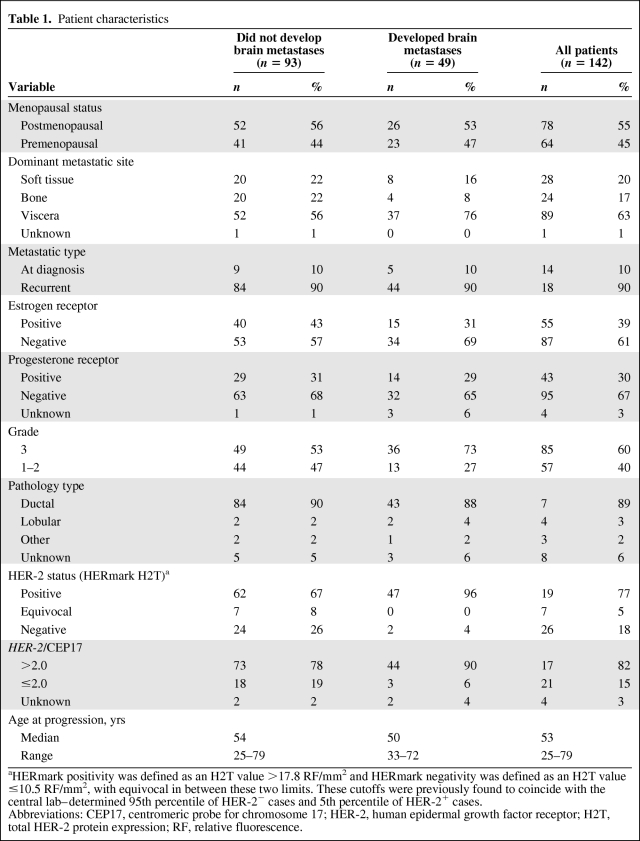
The study group included a consecutive series of HER-2+ (3+ as assessed by immunohistochemistry [IHC] or 2+ as assessed by IHC and HER-2+ as assessed by fluorescence in situ hybridization [FISH]) pathologically confirmed advanced breast cancer patients treated in nine Polish institutions between December 2000 and July 2010. All patients received at least one dose of trastuzumab with or without chemotherapy (typically taxanes, vinorelbine, or capecitabine) throughout the entire study period. The line of therapy during which trastuzumab was first administered was not recorded, although the median time from diagnosis of metastatic disease to the initiation of trastuzumab was 3.4 months (range, 0–49 months). This delay resulted from the fact that a substantial proportion of patients received trastuzumab as a second-line or subsequent line of therapy in the metastatic setting. Because the time from diagnosis of metastatic disease to the initiation of trastuzumab did not correlate with the time to brain metastasis (TTBM) (p = .7), this factor was not used for stratification. The majority of patients remained on trastuzumab treatment until progression, three patients terminated trastuzumab administration earlier as a result of the occurrence of excessive toxicity or personal decision, and 20 patients continued trastuzumab therapy beyond progression. Patients were identified through computerized hospital systems, protocol enrollment lists, or manual search. All slides were subjected to central pathology review. In total, 164 HER-2+ advanced breast cancer patients were identified initially, 22 of whom were subsequently removed from the analysis because of pre-existing brain metastases (n = 6) or because they received trastuzumab in the adjuvant setting (n = 16; eight of these also received trastuzumab for metastatic disease), leaving a study cohort of 142 patients (Table 1).
Table 1.
Patient characteristics
aHERmark positivity was defined as an H2T value >17.8 RF/mm2 and HERmark negativity was defined as an H2T value ≤10.5 RF/mm2, with equivocal in between these two limits. These cutoffs were previously found to coincide with the central lab–determined 95th percentile of HER-2− cases and 5th percentile of HER-2+ cases.
Abbreviations: CEP17, centromeric probe for chromosome 17; HER-2, human epidermal growth factor receptor; H2T, total HER-2 protein expression; RF, relative fluorescence.
The following information was extracted from the medical records: date of diagnosis of breast cancer, previous local and systemic therapy, date and type of first progression (local, regional, distant), dominant site of metastatic disease (soft tissue, bone, viscera), date of diagnosis of brain metastasis, dates on which trastuzumab was received, date of first progression while on trastuzumab therapy, and date of death or last follow-up visit. For tumors involving more than one category, the dominant site of distant disease was classified by the category associated with the worst prognosis, irrespective of the extent of involvement, in the following order of increasing gravity: soft tissue, bones, viscera. Because of the retrospective nature of this study, tumor staging was performed using the American Joint Committee on Cancer/Union for International Cancer Control classification from 1997. The brain metastases included radiographically confirmed (computed tomography or magnetic resonance imaging) parenchymal brain lesions. No screening for occult brain lesions was performed; therefore, all metastases were symptomatic or detected accidentally. Follow-up information was extracted from medical records and tumor registries. Because this analysis was based on a retrospective analysis of medical records, patient consent was not sought.
HER-2+ status was initially determined using semiquantitative IHC at the institutions participating in the study. HER-2 gene copy number assessment using FISH was performed centrally at the Department of Biology and Genetics, Medical University of Gdańsk. Gene amplification was defined as a FISH ratio (HER-2/centromeric probe for chromosome 17 [CEP17] ratio) >2.0. H2T was measured using the HERmark assay as described by Lipton et al. [33] and Larson et al. [35], with units of relative fluorescence per mm2 of invasive tumor (RF/mm2 tumor). Expression of estrogen receptor (ER) and progesterone receptor (PgR) was determined using IHC, with 10% nuclear staining considered as a positive result.
The median follow-up times were 68 months (range, 7–144 months) from the initial diagnosis of breast cancer, 34 months (range, 4–121 months) from the first occurrence of relapse, and 29 months (range, 1–115 months) from the initiation of trastuzumab-containing therapy. The median time from the initial diagnosis to first relapse was 22 months (range 0–103 months) and the median duration of trastuzumab therapy was 10 months (range, 1–115 months).
TTBM was calculated from the initiation of trastuzumab-containing treatment to the diagnosis of brain metastasis or was censored at the end of follow-up or death. The OS time was calculated from the initiation of trastuzumab-containing treatment to death (from any cause) or was censored at the end of follow-up. The Kaplan–Meier method was used to estimate the probability of brain metastases over time. p-values were calculated for the univariate analysis using the log-rank test with stratification where indicated. Cox models were used for multivariate analysis with stratification where indicated. Cox models were also used to estimate the hazard ratio (HR) and its confidence interval (CI). In the multivariate Cox models and in the univariate Cox model assessing this particular variable, time to non-brain progression was used as a time-dependent variable to examine the effect of other types of progression on the risk for brain metastases. Analyses controlling for the competing risks of death (on brain relapse and disease recurrence at all other sites) were performed by the method of subdistribution of competing risks as described by Fine and Gray [36]. p-values <.05 were considered significant. Statistical analyses were prespecified to the extent possible in a statistical analysis plan and were performed independently by separate teams at Monogram Biosciences, Inc. (South Francisco, CA) and International Drug Development Institute (IDDI), Inc. (Louvain-la-Neuve, Belgium). Any discrepancies were resolved by agreement among the clinical team in Poland and the statistical teams at Monogram and IDDI.
Results
Characteristics and Outcomes of Study Cohort
In total, 49 of 142 patients (35%) developed symptomatic brain relapse. Among those 49 patients, the median TTBM was 13 months (95% CI, 9–18 months). After the start of trastuzumab treatment, in 20 patients brain metastasis occurred at the time of first metastatic progression, 17 of whom developed brain relapse during trastuzumab treatment. The remaining 29 patients developed brain relapse after discontinuation of trastuzumab treatment. The cumulative risks for developing brain relapse at 1, 2, and 3 years from the initiation of trastuzumab-containing treatment were 19%, 30%, and 46%, respectively (95% CI, 12%–25%, 22%–39%, and 34%–58%, respectively). The median OS time from the initiation of trastuzumab therapy in the overall population was 32 months (95% CI, 28–43 months), 28 months (95% CI, 16–32 months), and 40 months (95% CI, 28–66 months) in the subgroups of patients who did and did not develop brain metastasis, respectively.
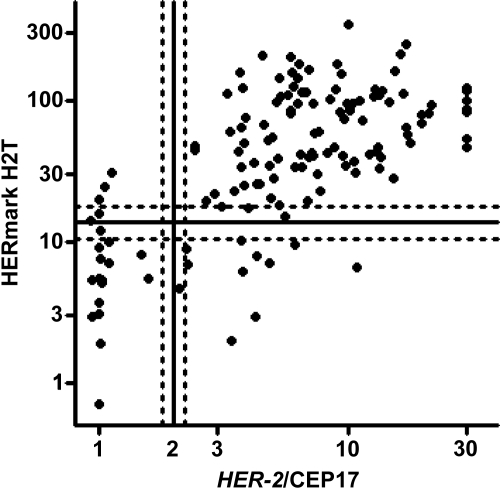
HER-2 amplification was found in 117 of the 138 cases analyzable using FISH (85%). H2T was 86% concordant (κ = 0.55) with the HER-2/CEP17 ratio, considering negative, equivocal, and positive categories for both H2T and the HER-2/CEP17 ratio (Fig. 1).
Figure 1.
Relationship between quantitative HER-2 protein level (H2T) as assessed by HERmark and HER-2 as assessed by fluorescence in situ hybridization (FISH). Cutoffs for HER-2 positivity by FISH and HERmark are indicated by solid lines. FISH and HERmark equivocal zones (HER-2/CEP17 ratio of 1.8–2.2 for FISH; 10.5–17.8 RF/mm2 for HERmark) are bounded by dotted lines. Concordance between H2T assessed by HERmark and the HER-2/CEP17 ratio assessed by FISH was 86% (κ = 0.55).
Abbreviations: CEP17, centromeric probe for chromosome 17; HER-2, human epidermal growth factor receptor 2; RF, relative fluorescence.
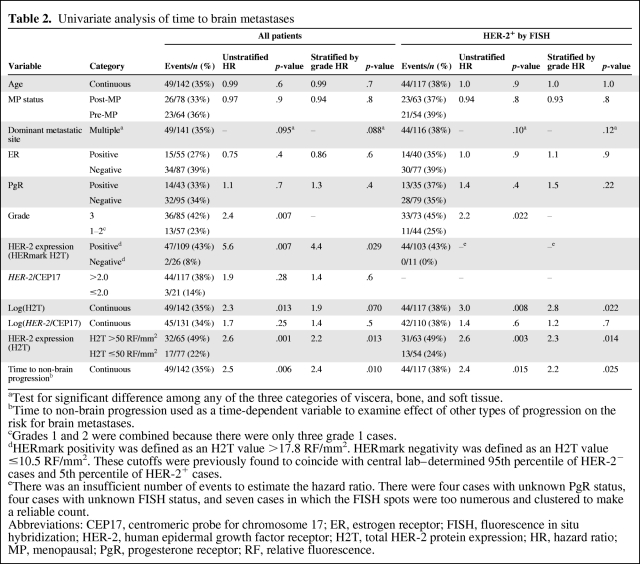
Determinants of TTBM
Twelve correlates of TTBM were explored (Table 2), including: (a) commonly used clinical variables (age, menopausal status, dominant site of metastatic disease, ER and PgR levels, tumor grade, HER-2 status by conventional FISH), (b) time to non-brain progression, and (c) the novel measurement of continuous HER-2 protein expression H2T, considered as a categorical variable using a specific cutoff and also as a continuous variable. A higher H2T (assessed either as a continuous variable or as a categorical variable using defined cutoffs) was significantly correlated with a shorter TTBM in the entire HER-2+ patient population, defined as HER-2+ either by IHC 3+ or by FISH ratio >2.0. Other variables correlated with TTBM included tumor grade and time from initiation of trastuzumab therapy to non-brain first progression. The HR for the previously defined positive H2T versus negative H2T groups (Table 2 footnote) was 5.6 (p = .007). However, in this study, the negative H2T group was small (n = 26). The best discriminating H2T cutoff value was found to be 50 RF/mm2, with an HR of 2.6 (p = .001). Of note, this value was close to the median H2T value of 58 RF/mm2. Continuous H2T was also significantly correlated with TTBM (p = .013), indicating a proportional increase in the risk for brain metastases across the entire range of H2T measurements. In contrast to H2T, neither the cutoff of a HER-2/CEP17 ratio of 2.0 nor a continuous HER-2/CEP17 ratio correlated significantly with TTBM (p = .28 and .25, respectively).
Table 2.
Univariate analysis of time to brain metastases
aTest for significant difference among any of the three categories of viscera, bone, and soft tissue.
bTime to non-brain progression used as a time-dependent variable to examine effect of other types of progression on the risk for brain metastases.
cGrades 1 and 2 were combined because there were only three grade 1 cases.
dHERmark positivity was defined as an H2T value >17.8 RF/mm2. HERmark negativity was defined as an H2T value ≤10.5 RF/mm2. These cutoffs were previously found to coincide with central lab–determined 95th percentile of HER-2− cases and 5th percentile of HER-2+ cases.
eThere was an insufficient number of events to estimate the hazard ratio. There were four cases with unknown PgR status, four cases with unknown FISH status, and seven cases in which the FISH spots were too numerous and clustered to make a reliable count.
Abbreviations: CEP17, centromeric probe for chromosome 17; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor; H2T, total HER-2 protein expression; HR, hazard ratio; MP, menopausal; PgR, progesterone receptor; RF, relative fluorescence.
As noted above, both H2T and tumor grade were univariate correlates of TTBM. To confirm that H2T impacted TTBM in addition to grade, the analyses were repeated with tumor grade as a stratification factor. Stratifying for grade, the HR for H2T at a cutoff of 50 RF/mm2 remained significant (HR, 2.2; p = .013), although the correlation of a continuous H2T with TTBM in the entire population was only trending (p = .070).
A competing risks analysis was performed to confirm that the occurrence of death was not impeding the ability to accurately measure the correlation of H2T with TTBM. Controlling for death, H2T remained a significant correlate of TTBM using both the H2T = 50 RF/mm2 cutoff (HR, 2.7; p = .0009) and a continuous H2T (HR, 2.7; p = .0066) with HRs similar to or slightly higher than those calculated without controlling for death (Table 2) (HRs of 2.6 and 2.3, respectively).
H2T assessed either as a continuous variable or as a categorical variable using defined cutoffs was not correlated with OS in our patient population.
TTBM in the HER-2+ Population as Assessed by FISH
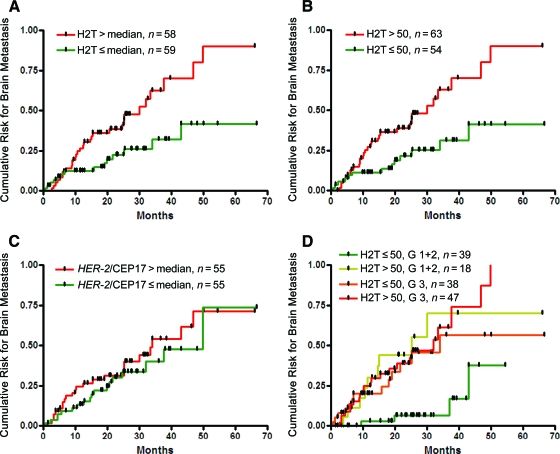
Because of the lack of central HER-2 IHC assessment and the possibility that some of the cases included might have been false positive for HER-2 by IHC, additional analyses were performed in the subset of 117 patients classified by central laboratory testing as HER-2+ by FISH (Table 2). In this group, H2T (assessed as a continuous variable and using defined cutoffs, with or without stratification by tumor grade) was significantly correlated with TTBM, whereas a continuous HER-2/CEP17 ratio was not (p = 0.4). Within the HER-2+ population assessed by FISH, patients with tumors expressing HER-2 above the median (H2T, 58 RF/mm2) were more than twofold more likely to develop brain metastases than those with a HER-2 level below the median—HRs of 2.4 (p = .005) and 2.2 (p = .019) without and with grade stratification, respectively (Fig. 2A). An even larger HR was seen for the H2T cutoff value of 50 RF/mm2, HRs of 2.6 (p = .003) and 2.3 (p = .014) without and with grade stratification, respectively (Fig. 2B). In contrast, patients with tumors determined to have HER-2 amplification above or below the median (HER-2/CEP17 = 6.9) had a similar likelihood of developing brain metastases (HR, 1.3; p = 0.4) both with and without grade stratification (Fig 2C). Similar results were also observed using other FISH HER-2/CEP17 cutoffs (data not shown).
Figure 2.
Time to brain metastasis according to quantitative HER-2 protein level (H2T), the HER-2/CEP17 ratio as assessed by fluorescence in situ hybridization (FISH), and grade in the subset that was HER-2+ by FISH. (A): H2T above the median (HR, 2.4; p = .005); (B): H2T >50 RF/mm2 (HR, 2.6; p = .003); (C): HER-2/CEP17 ratio as assessed by FISH above versus below the median (HR, 1.3; p = .4); (D): H2T low–grade 1–2 versus three other subsets (HR, 0.17; p = .0001).
Abbreviations: CEP17, centromeric probe for chromosome 17; G, grade; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; RF, relative fluorescence.
Effect of Tumor Grade on H2T Correlation with TTBM
Because tumor grade was found to be a significant correlate of TTBM on univariate analysis, and it had some effect on the HR CIs for H2T when used as a stratification factor, we separately examined the correlation of H2T with TTBM in the subsets of patients with grade 1–2 and grade 3 tumors. The correlation between H2T and TTBM was stronger in the grade 1–2 subset, consistent with an interaction between H2T and tumor grade (p = .025). TTBM for the four subgroups defined by the H2T cutoff of 50 RF/mm2 and two grade categories (combined grades 1–2 versus grade 3) showed similar outcomes for three of the groups (H2T low–grade 3, H2T high–grade 1–2, H2T high–grade 3) as compared to that of the H2T low–grade 1–2 subset, which had a significantly lower likelihood of developing brain metastases (log-rank p = .0012 for comparison of the four subgroups) (Fig. 2D). A univariate Cox model estimated an HR of 0.17 for the H2T low–grade 1–2 patients compared with patients in the three other groups (p = .0001). Even though the log-rank comparison of the four subgroups (with three degrees of freedom) was also significant (p = .0012), this result should be interpreted cautiously given the multiple comparisons that are possible among these four subsets. To assess whether this was a general phenomenon across the entire range of H2T values, we examined the correlation between a continuous H2T level and TTBM within the two subgroups of grades 1–2 and grade 3 patients. No difference was found for the correlation between a continuous H2T and TTBM between the particular grade groups in the entire population (interaction p = .6); however, a trend toward a stronger correlation between H2T and TTBM was observed in the grade 1–2 patient subgroup than in the grade 3 patient subgroup in the subset of patients who were HER-2+ by FISH (interaction p = .10).
Impact of Progression Other Than Brain Metastases on TTBM
We previously demonstrated that a shorter time to relapse in HER-2+ advanced breast cancer patients was associated with a higher risk for developing brain metastases [24]. Similarly, in the current cohort, time to non-brain progression treated as a time-dependent variable was found to be significantly associated with TTBM on univariate analysis (Table 2). To further examine the correlation between other sites of progression and TTBM, multivariate models were fitted with TTBM as the outcome, H2T and the HER-2/CEP17 ratio as baseline variables, and progression other than brain metastasis as a time-dependent variable (Table 3). The models were stratified by tumor grade and ER and PgR status. H2T and the HER-2/CEP17 ratio were tested as continuous variables rather than using defined cutoffs to avoid potential overfitting associated with particular cutoffs. Time to non-brain progression was significantly associated with TTBM while controlling for the other variables. In the subset of patients who were HER-2+ by FISH, a continuous H2T level (HR, 3.3; p = .024) and time to non-brain progression (HR, 2.9; p = .0056) were correlated with TTBM, whereas HER-2/CEP17 was not.
Table 3.
Multivariate analysis of time to brain metastasis
aTime to non-brain progression used as a time-dependent variable to examine the effect of other types of progression on the risk for brain metastases.
Abbreviations: CEP17, centromeric probe for chromosome 17; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor; H2T, total HER-2 protein expression.
HER-2 Level Distribution by Dominant Metastatic Site
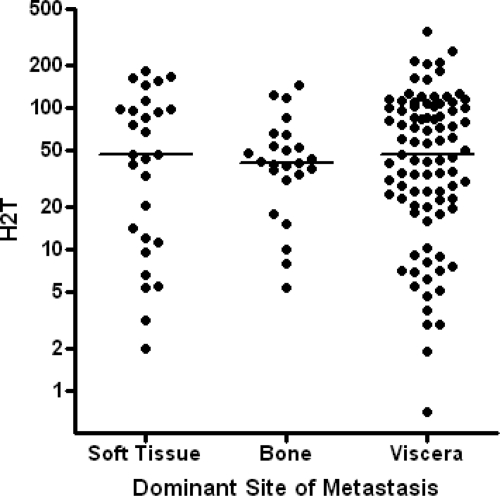
Following the observation that H2T was correlated with TTBM, we attempted to determine whether or not the occurrence of metastases at other sites was also correlated with H2T. Detailed site-specific follow-up was not available for other metastatic sites; however, the dominant site of metastasis was assessed for 141 of the 142 patients. The distributions of H2T measurements were not different among groups of patients based on dominant metastatic sites, including soft tissue, bone, and extracranial viscera (p = 0.9) (Fig. 3). In addition, no correlation was observed between the dominant metastatic site and TTBM (p = .1).
Figure 3.
Human epidermal growth factor receptor 2 protein level (H2T) in primary tumor for each of three dominant metastatic sites. No significant differences were apparent (p = .9, Kruskal-Wallis test). H2T is in units of relative fluorescence per mm2 tumor.
Discussion
This study is the first to demonstrate that the quantitative assessment of HER-2 expression in the primary tumor can be used to identify HER-2+ trastuzumab-treated advanced breast cancer patients at particularly high risk for developing brain metastases. Notably, in the multivariate model, H2T (quantitative HER-2 level), tumor grade, and time to distant progression were the only predictors of brain relapse, and only H2T and time to distant progression were statistically significant predictors of this event in the most stringently selected subset of HER-2+ patients as assessed by FISH. All other molecular and clinical factors, such as the HER-2/CEP17 ratio, HER-2 amplification, hormone receptor status, menopausal status, and age, were not statistically significant correlates of TTBM.
HER-2 overexpression is a well-recognized factor associated with a higher risk for brain metastases [3–5], and there is relatively high concordance for HER-2 status between the primary tumor and brain metastases within the same patient [37, 38]. The correlation between high HER-2 expression and a higher risk for brain metastases might be attributable to the specific biology of HER-2–overexpressing tumors [6]. This hypothesis is consistent with the historical “seed and soil” hypothesis of Paget, which suggested that the establishment of a metastasis in a distant organ favors tumor cells (the “seed”) with the greatest affinity for the growth-enhancing microenvironment of the target organ, providing a growth advantage to the seeds (the “soil”) [39].
Indeed, various members of the epidermal growth factor receptor (EGFR) family and their ligands have been shown to mediate breast cancer metastasis to the brain in a multistage process, including blood–brain barrier infiltration, extravasation, and brain colonization [7, 40]. For example, expression of heparin-binding epidermal growth factor, a ligand of EGFR, was shown to enhance the extravasation of cancer cells through nonfenestrated capillaries and to stimulate the growth of a variety of cells through autocrine or paracrine signaling [7]. In animal models, HER-2 overexpression was found to increase the outgrowth of metastatic tumor cells in the brain [40]. HER-2 may also interact with other members of the HER family to enable the establishment of brain metastases in patients with breast cancer [41]. The finding of this investigation that high levels of HER-2 expression correlate with a higher risk for brain metastases is consistent with these prior data.
HER-2 amplification is generally considered to be the main mechanism of HER-2 overexpression in breast cancer, and there is generally high concordance between HER-2 status by FISH testing (DNA) and HER-2 status by IHC testing (protein). However, the results of this study have shown that H2T as assessed using HERmark (continuous HER-2 level) is inversely correlated with TTBM, whereas HER-2 amplification as assessed using FISH (continuous HER-2/CEP17 ratio) is not predictive, although a similar trend was observed. There are several factors that may explain this observation. First, H2T is a quantitative measurement of the functional protein, whereas the HER-2/CEP17 ratio is a numeric count of HER-2 gene amplification (DNA). The biologic regulation of HER-2 expression in tumor cells is complex, and HER-2 amplification may not always correlate quantitatively with HER-2 expression at the receptor level as a result of either post-transcriptional or post-translational modifications. Second, the DNA amplicon detected by the HER-2 FISH probe contains a number of other biologically significant genes, such as the gene encoding topoisomerase II-α (TOP2A), that may have an impact on the clinical outcome of breast cancer patients, including metastasis to the brain. Finally, the difference in assay design and thus the ability to provide an accurate quantitative measurement of HER-2 may also play a role. The HERmark assay is able to measure a continuous distribution of HER-2 expression extending over a large dynamic range corresponding to approximately 25,000 to two million receptors/cell, based on a study of control breast cancer cell lines [32]. Accurate measurement of HER-2 signals in the HER-2 FISH test may become difficult, particularly in tumors with a high copy number, for which the fluorescent HER-2 signals may present as clusters instead of distinct individual signals for numeric counting of HER-2.
Higher H2T values were previously shown to be associated with a better response to trastuzumab and longer time to progression in advanced breast cancer patients [33, 34]. In the current study, high H2T was associated with a shorter TTBM, whereas this was not true for extracranial sites of metastasis. Presumably, this differential biological effect of H2T is a result of the inability of trastuzumab to penetrate the central nervous system [16–24]. One might imagine that better control of extracranial disease by trastuzumab may merely provide more time for the clinical manifestation of brain relapse; however, analyses performed to account for progressions at other sites as competing events confirmed a significant correlation between H2T (considered as continuous variable) and TTBM.
Importantly, all patients in this study were administered trastuzumab in the metastatic setting. Although trastuzumab does not effectively penetrate the blood–brain barrier, it may impact the development of brain metastases through indirect mechanisms. It is therefore unknown whether or not our findings are applicable to the population of trastuzumab-naïve patients.
Conclusion
The correlation between a quantitative measurement of HER-2 expression (H2T) and the risk for brain metastasis in trastuzumab-treated advanced HER-2+ breast cancer patients suggests that H2T assessment may prove useful in the identification of patients who would benefit from more personalized preventive and therapeutic strategies in this otherwise high-risk population. Currently several new compounds with potential prophylactic or therapeutic activity for brain metastases are undergoing clinical evaluation. In contrast to trastuzumab, small molecule tyrosine kinase inhibitors do traverse the blood–brain barrier, although access to brain metastases may still be impeded [42]. Lapatinib has shown promise at preventing the emergence of brain metastases in preclinical breast cancer models [43] and has shown some effectiveness in treating brain metastases in the clinical setting [44]. In a randomized phase III study, the addition of lapatinib to capecitabine in patients who progressed after trastuzumab therapy was associated with a lower rate of symptomatic brain relapse [45]. Other agents, such as pazopanib, have shown activity in brain metastasis prevention in mice injected with HER-2–overexpressing cell lines [46]. As HER-2–directed small molecule agents gain regulatory approval for indications including primary breast cancer, quantitative measurements of HER-2 expression may prove useful in guiding patient management.
Because of its retrospective design, limited sample size, lack of a trastuzumab-untreated control arm, and lack of central IHC measurements to initially exclude false-positive HER-2 cases, we consider these results as preliminary, hypothesis generating, and warranting confirmation in future studies. The next step in our study, therefore, is an independent validation of the results presented here.
Additional studies are needed to understand the clinical utility of these findings. There is also a need for identifying additional clinical and biological factors that could be used to detect breast cancer patients at risk for brain metastasis and to develop treatment strategies to effectively combat this serious complication.
Acknowledgments
The following investigators contributed to this study: Janusz Limon, Anita Matyskiel and Katarzyna Sosińska-Mielcarek, Medical University, Gdańsk, Poland; Bartłomiej Grala, Military Institute of Medicine, Warsaw, Poland; Robert Wiraszka, Regional Hospital, Radom, Poland; Franciszek Szubstarski, Lublin Oncology Center, Lublin, Poland; Konstanty Korski, Great Poland Cancer Center, Poznan, Poland; Maria Chosia, Medical University, Szczecin, Poland.
This research was conducted in cooperation with Monogram Biosciences, the company manufacturing the HERmark assays, and some authors are Monogram Biosciences employees.
M.B. is currently affiliated with Cepheid, Sunnyvale, CA.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Jacek Jassem, Renata Duchnowska, Wojciech Biernat, Barbara Szostakiewicz, Jeff Sperinde, Fanny Piette, Mojgan Haddad, Agnes Paquet, Bogumiła Czartoryska-Arłukowicz, Piotr Wysocki, Tomasz Jankowski, Barbara Radecka, Małgorzata Foszczyńska-Kłoda, Maria Litwiniuk, Sylwia Dȩbska, Jodi Weidler, Weidong Huang, Marc Buyse, Michael Bates
Provision of study material or patients: Jacek Jassem, Renata Duchnowska, Wojciech Biernat, Barbara Szostakiewicz, Bogumiła Czartoryska-Arłukowicz, Piotr Wysocki, Tomasz Jankowski, Barbara Radecka, Małgorzata Foszczyńska-Kłoda, Maria Litwiniuk, Sylwia Dȩbska
Collection and/or assembly of data: Jacek Jassem, Renata Duchnowska, Wojciech Biernat, Barbara Szostakiewicz, Jeff Sperinde, Mojgan Haddad, Agnes Paquet, Yolanda Lie, Bogumiła Czartoryska-Arłukowicz, Piotr Wysocki, Tomasz Jankowski, Barbara Radecka, Małgorzata Foszczyńska-Kłoda, Maria Litwiniuk, Jodi Weidler, Weidong Huang
Data analysis and interpretation: Jacek Jassem, Renata Duchnowska, Wojciech Biernat, Barbara Szostakiewicz, Jeff Sperinde, Fanny Piette, Mojgan Haddad, Agnes Paquet, Bogumiła Czartoryska-Arłukowicz, Piotr Wysocki, Tomasz Jankowski, Barbara Radecka, Małgorzata Foszczyńska-Kłoda, Maria Litwiniuk, Sylwia Dȩbska, Jodi Weidler, Weidong Huang, Marc Buyse, Michael Bates
Manuscript writing: Jacek Jassem, Renata Duchnowska, Jeff Sperinde, Fanny Piette, Agnes Paquet, Jodi Weidler, Weidong Huang, Marc Buyse
Final approval of manuscript: Jacek Jassem, Renata Duchnowska, Wojciech Biernat, Barbara Szostakiewicz, Jeff Sperinde, Fanny Piette, Mojgan Haddad, Agnes Paquet, Yolanda Lie, Bogumiła Czartoryska-Arłukowicz, Piotr Wysocki, Tomasz Jankowski, Barbara Radecka, Małgorzata Foszczyńska-Kłoda, Maria Litwiniuk, Sylwia Dȩbska, Jodi Weidler, Weidong Huang, Marc Buyse, Michael Bates
References
- 1.Tsukada Y, Fouad A, Pickren JW, et al. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 3.Hicks DG, Short SM, Prescott NL, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 4.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Angulo AM, Cristofanilli M, Strom EA, et al. Central nervous system metastases in patients with high-risk breast carcinoma after multimodality treatment. Cancer. 2004;101:1760–1766. doi: 10.1002/cncr.20530. [DOI] [PubMed] [Google Scholar]
- 6.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 7.Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen DX, Bos PD, Massagué J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Burstein HJ, Keshaviah A, Baron AD, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: The trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 11.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: The M77001 Study Group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 12.Schaller G, Fuchs I, Gonsch T, et al. Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol. 2007;25:3246–3250. doi: 10.1200/JCO.2006.09.6826. [DOI] [PubMed] [Google Scholar]
- 13.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2–overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 14.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18:2349–2351. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 15.Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18:23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 16.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 17.Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer. 2004;40:379–382. doi: 10.1016/j.ejca.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai R, Dang CT, Malkin MG, et al. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101:810–816. doi: 10.1002/cncr.20418. [DOI] [PubMed] [Google Scholar]
- 20.Lower EE, Drosick RD, Blau R, et al. Increased rate of brain metastases with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer. 2003;4:114–119. doi: 10.3816/cbc.2003.n.016. [DOI] [PubMed] [Google Scholar]
- 21.Burstein HJ, Lieberman G, Slamon DJ, et al. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol. 2005;16:1772–1777. doi: 10.1093/annonc/mdi371. [DOI] [PubMed] [Google Scholar]
- 22.Stemmler HJ, Kahlert S, Siekiera W, et al. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15:219–225. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Gori S, Rimondini S, De Angelis V, et al. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: Incidence, survival, and risk factors. The Oncologist. 2007;12:766–773. doi: 10.1634/theoncologist.12-7-766. [DOI] [PubMed] [Google Scholar]
- 24.Duchnowska R, Dziadziuszko R, Czartoryska-Arłukowicz B, et al. Risk factors for brain relapse in HER2-positive metastatic breast cancer patients. Breast Cancer Res Treat. 2009;117:297–303. doi: 10.1007/s10549-008-0275-z. [DOI] [PubMed] [Google Scholar]
- 25.Nam BH, Kim SY, Han HS, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20. doi: 10.1186/bcr1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: The importance of HER-2 status. Cancer. 2008;112:2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 27.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 28.Park IH, Ro J, Lee KS, et al. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20:56–62. doi: 10.1093/annonc/mdn539. [DOI] [PubMed] [Google Scholar]
- 29.Church DN, Modgil R, Guglani S, et al. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol. 2008;31:250–254. doi: 10.1097/COC.0b013e31815a43c4. [DOI] [PubMed] [Google Scholar]
- 30.Metro G, Sperduti I, Russillo M, et al. Clinical utility of continuing trastuzumab beyond brain progression in HER-2 positive metastatic breast cancer. The Oncologist. 2007;12:1467–1469. doi: 10.1634/theoncologist.12-12-1467. [DOI] [PubMed] [Google Scholar]
- 31.Duchnowska R, Jassem J, Thorat M, et al. Gene expression analysis for prediction of early brain metastasis in HER2-positive breast cancer patients. Presented at the American Society of Clinical Oncology Annual Meeting; May 30 to June 3, 2008; Chicago, IL. [Google Scholar]
- 32.Shi Y, Huang W, Tan Y, et al. A novel proximity assay for the detection of proteins and protein complexes: Quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn Mol Pathol. 2009;18:11–21. doi: 10.1097/PDM.0b013e31818cbdb2. [DOI] [PubMed] [Google Scholar]
- 33.Lipton A, Köstler WJ, Leitzel K, et al. Quantitative HER2 protein levels predict outcome in fluorescence in situ hybridization-positive patients with metastatic breast cancer treated with trastuzumab. Cancer. 2010;116:5168–5178. doi: 10.1002/cncr.25430. [DOI] [PubMed] [Google Scholar]
- 34.Toi M, Sperinde J, Huang W, et al. Differential survival following trastuzumab treatment based on quantitative HER2 expression and HER2 homodimers in a clinic-based cohort of patients with metastatic breast cancer. BMC Cancer. 2010;10:56. doi: 10.1186/1471-2407-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson JS, Goodman LJ, Tan Y, et al. Analytical validation of a highly quantitative, sensitive, accurate, and reproducible assay (HERmark) for the measurement of HER2 total protein and HER2 homodimers in FFPE breast cancer tumor specimens. Patholog Res Int. 2010;2010:814176. doi: 10.4061/2010/814176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 37.Lear-Kaul KC, Yoon HR, Kleinschmidt-DeMasters BK, et al. HER-2/neu status in breast cancer metastases to the central nervous system. Arch Pathol Lab Med. 2003;127:1451–1457. doi: 10.5858/2003-127-1451-NSIBCM. [DOI] [PubMed] [Google Scholar]
- 38.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 40.Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 41.Da Silva L, Simpson PT, Smart CE, et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010;12:R46. doi: 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gril B, Palmieri D, Bronder JL, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 46.Gril B, Palmieri D, Qian Y, et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin Cancer Res. 2011;17:142–153. doi: 10.1158/1078-0432.CCR-10-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]