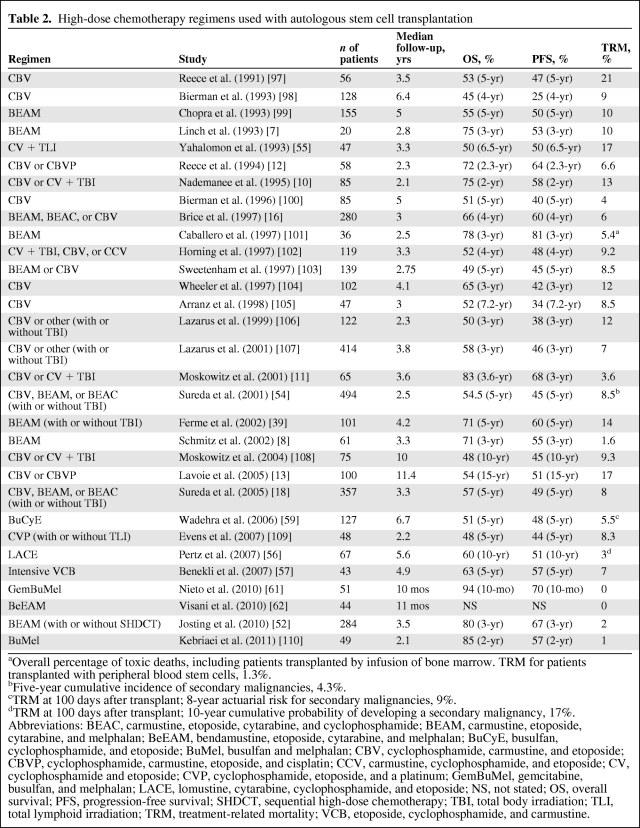
Table 2.
High-dose chemotherapy regimens used with autologous stem cell transplantation
aOverall percentage of toxic deaths, including patients transplanted by infusion of bone marrow. TRM for patients transplanted with peripheral blood stem cells, 1.3%.
bFive-year cumulative incidence of secondary malignancies, 4.3%.
cTRM at 100 days after transplant; 8-year actuarial risk for secondary malignancies, 9%.
dTRM at 100 days after transplant; 10-year cumulative probability of developing a secondary malignancy, 17%.
Abbreviations: BEAC, carmustine, etoposide, cytarabine, and cyclophosphamide; BEAM, carmustine, etoposide, cytarabine, and melphalan; BeEAM, bendamustine, etoposide, cytarabine, and melphalan; BuCyE, busulfan, cyclophosphamide, and etoposide; BuMel, busulfan and melphalan; CBV, cyclophosphamide, carmustine, and etoposide; CBVP, cyclophosphamide, carmustine, etoposide, and cisplatin; CCV, carmustine, cyclophosphamide, and etoposide; CV, cyclophosphamide and etoposide; CVP, cyclophosphamide, etoposide, and a platinum; GemBuMel, gemcitabine, busulfan, and melphalan; LACE, lomustine, cytarabine, cyclophosphamide, and etoposide; NS, not stated; OS, overall survival; PFS, progression-free survival; SHDCT, sequential high-dose chemotherapy; TBI, total body irradiation; TLI, total lymphoid irradiation; TRM, treatment-related mortality; VCB, etoposide, cyclophosphamide, and carmustine.