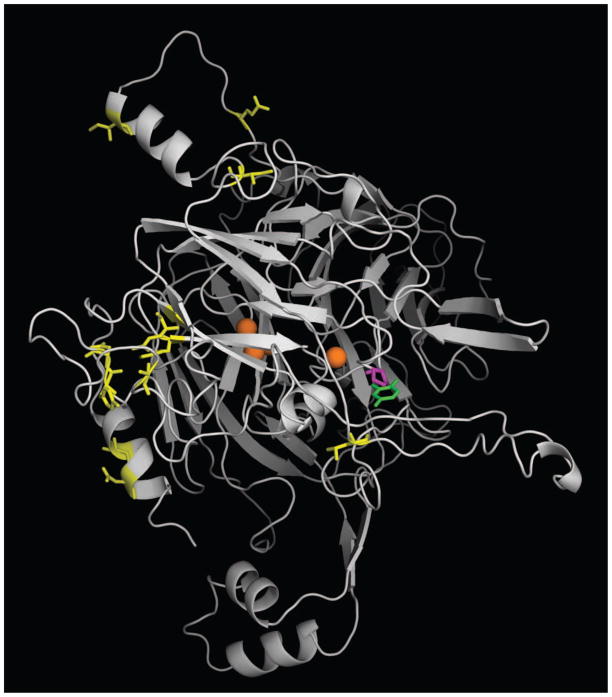
Figure 2.
Homology model of AgMCO2A. The crystal structure of T. versicolor laccaseIIIb complexed with a laccase substrate, 2,5-xylidine, was used as a template for generating a homology model of AgMCO2A. Coppers are shown as orange spheres. 2,5-xylidine is shown in green. Residues corresponding to conserved differences between A and B isoforms (Figure 1) are shown in yellow. Residue 633, which is a proline in AgMCO2A and a glutamine in AgMCO2B, is shown in magenta. Note that positions corresponding to conserved differences are outside of the predicted substrate binding pocket, but residue 633 is within the predicted substrate binding pocket.