Summary
Purpose
How long after starting a new medication must a patient go without seizures before they can be regarded as seizure free? A recent ILAE task force proposed using a “Rule of Three” as an operational definition of seizure freedom, according to which a patient should be considered seizure-free following an intervention after a period without seizures has elapsed equal to three times the longest pre-intervention inter-seizure interval over the previous year. This rule was motivated in large part by statistical considerations advanced in a classic 1983 paper by Hanley and Lippman-Hand. However, strict adherence to the statistical logic of this rule generally requires waiting much longer than recommended by the ILAE task force. Therefore, we set out to determine whether an alternative approach to the Rule of Three might be possible, and under what conditions the rule may be expected to hold or would need to be extended.
Methods
Probabilistic modeling and application of Bayes’ rule.
Key Findings
We find that an alternative approach to the problem of inferring seizure freedom supports using the Rule of Three in the way proposed by the ILAE in many cases, particularly in evaluating responses to a first trial of anti-seizure medication, and to favorably-selected epilepsy surgical candidates. In cases where the a priori odds of success are less favorable, our analysis requires longer seizure-free observation periods before declaring seizure freedom, up to six times the average pre-intervention insterseizure interval. The key to our approach is to take into account not only the time elapsed without seizures but also empirical data regarding the a priori probability of achieving seizure freedom conferred by a particular intervention.
Significance
In many cases it may be reasonable to consider a patient seizure free after they have gone without seizures for a period equal to three times the pre-intervention inter-seizure interval, as proposed on pragmatic grounds in a recent ILAE position paper, though in other commonly encountered cases a waiting time up to six times this interval is required. In this work we have provided a coherent theoretical basis for modified criterion for seizure freedom, which we call the “Rule of Three-To-Six”.
Keywords: Bayes’ rule, ILAE, Epilepsy, Refractory, Statistical prediction
Introduction
While seizure freedom is always the goal of initiating antiepileptic drug (AED) therapy in patients with epilepsy, identifying when this goal has been achieved can be problematic. A common challenge for epileptologists lies in determining what constitutes an adequate amount of time without seizures following an intervention such as the start of a new medication for a patient to be considered seizure free. This determination has important implications for counseling patients about when to resume risky activities, and for public policy such as how long patients must go without seizures before returning to driving. A recent ILAE task force proposed that a patient should be considered “seizure free” in response to a new anti-seizure treatment (e.g. medication or surgery) once they have gone without a seizure for at least three times the duration of their longest pre-intervention inter-seizure interval in the preceding 12 months (Kwan et al., 2009). Inspiration for this proposed working definition of seizure freedom is credited to a statistical principle known as the “Rule of Three”, proposed several decades ago as a generic statistical rule of thumb for reasoning about “zero numerators”, that is, for inferring from the fact that no adverse events have occurred so far the probability that an adverse event may yet occur at some future time (Hanley & Lippman-Hand, 1983; Jovanovic & Levy, 1997). However, as acknowledged by the ILAE task force, the proposed application towards the determination of seizure freedom entails a compromise, dictated by practical considerations rather than following strictly from the logic embodied in the original statistical formulation of the Rule of Three (Kwan et al., 2009).
Because the Rule of Three has not to our knowledge been applied before to the problem of determination of seizure freedom, and because the rule in its original formulation is not widely known among epileptologists, our first goal in this paper will be to provide an accessible mathematical derivation and an explanation of its literal meaning and implications. We shall see that it relies on a type of “worst case scenario” reasoning which, followed strictly, requires waiting much longer than the period proposed in ILAE task force definition of seizure freedom, in some cases for many years, hence the original Rule of Three apparently requires modification to be of practical clinical use. Hereafter, we will refer to the original statistical formulation and its attendant statistico-logical implications as the “Classical Rule of Three”. Our second goal in this paper will be to propose a set of probabilistic considerations which do support the ILAE’s proposed pragmatic adaptation of the Classical Rule of Three, at least for many cases encountered in clinical practice, thus placing the rule on more solid theoretical grounds. We shall demonstrate that for many cases commonly encountered in practice, a waiting period of three times the pre-intervention interseizure interval is adequate, whereas in other cases as long as six interseizure intervals is required. Consequently, we refer to our final justified-and-extended version of the Classical Rule of Three, and the attendant probabilistic considerations behind our formulation, as the “Rule of Three-To-Six”.
The fulcrum of our approach is the recognition that information available before initiating an intervention can be informative in interpreting the response to an intervention so far, whereas such information is ignored by the Classical Rule of Three. For example, in medication-naive adult epilepsy patients, it is well-known that roughly 50-70% become “seizure free” in response to AED therapy, whereas for patients who have already “failed” 1-2 previous AEDs, the probability of achieving seizure freedom with subsequent AED trials is known to be low, around 5-10% (Kwan & Brodie, 2000, 2001). Similarly, certain carefully selected patients with lesional epilepsy can be quoted an approximately 80% probability of achieving seizure freedom with surgery (McIntosh et al, 2001). Such pre-intervention probabilities are routinely used in counseling patients contemplating new medical or surgical interventions. We therefore propose a simple statistical model that allows such estimates of the a priori probability of seizure freedom to be combined, via Bayes’ rule, with the time without a seizure since starting medication, to yield an informed estimate of the probability that a patient will remain seizure free. This model leads to a principled probabilistic justification for the ILAE’s proposed working definition of seizure freedom, rescuing it from the shortcomings of the Classical Rule of Three.
Methods
In the derivations given below of the Classical Rule of Three and our modification of it, we will make the simplifying assumption that seizures occur at random times, with a patient-specific underlying rate, r, or, equivalently, with an average inter-seizure interval of τ = 1/r. That is, we assume the probability that a seizure occurs during any very small time increment, δt, is constant, and equal to the length of this interval times the seizure rate, rδt. From this elementary assumption, an expression can be derived for the probability that any specified number of seizures N(t) may occur within a time interval of length t (see Supplemental Material), namely
This mathematical model is known as a Poisson process (Papoulis, 1984). The notation Pr(N(t) = k∣r) is read, the probability that k seizures occur within an interval of time t, given that the patient’s underlying seizure rate is r.
In the remainder of this paper, we will be chiefly interested in the case in which no seizures occur over a given observation period following an intervention. More precisely, we are interested in the probability that no seizures occur over an interval of length t, hence P(N(t) = 0∣r), or equivalently, in the probability that the time to the next seizure, Δ t, lies beyond the specified interval, Pr (Δ t > t∣r). Substituting into the Poisson process equation above, we get simply
Hence, if seizure “arrival times” obey a Poisson process model, then the probability distribution of interseizure intervals is a simple exponential decay function.
Many processes involving recurrent random events can be reasonably approximated by Poisson process models, though it must be realized that such models treat the intervals between events as statistically independent, and hence cannot describe more complex temporal features characteristic of some cases of epilepsy such as systematic clustering or diurnal variation, which may play important roles in the management of some patients’ seizures (Balish et al., 1991; Haut et al., 2005). Expected effects of deviations from this simple model on the results of the following analysis are addressed in the Discussion section and Supplemental Material.
Results
Formulation of the Classical Rule of Three
Figure 1A illustrates a series of random seizure occurrence times generated by the Poisson model over a five year period for five hypothetical patients with rates ranging from 1 seizure / week to 1 seizure / year. Visual inspection of these examples suggests what statistical analysis confirms: while seizure free intervals much longer than a patient’s average are uncommon, occasional prolonged seizure free intervals do occur by chance. Figure 1B further illustrates this phenomenon and its implications by simulating seizure occurrences in a single patient over a five year period. In this model, the patient’s initial seizure rate is 1 seizure / 3 months. Thirty-six months into the simulation, an intervention is initiated, and the patient becomes seizure free, i.e. the seizure rate in the model is reduced to 0 seizures/month. While the patient’s newfound seizure freedom seems obvious when considering seizure activity across the entire five year simulation in Figure 1B, one is challenged to identify the time point after medication onset at which seizure freedom has unequivocally been achieved. This task is particularly difficult in light of the patient’s rather long seizure free period at the beginning of the simulation, an inter-seizure interval that, while much longer than the patient’s average, is still within the distribution of possible inter-seizure intervals. Thus, the question naturally arises: What duration of seizure freedom is necessary before the patient or doctor counseling the patient can conclude, with reasonable certainty, that the intervention has rendered the patient seizure free?
Figure 1.
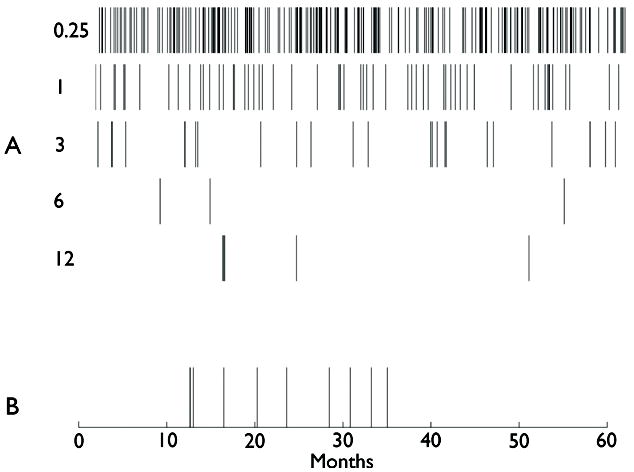
Illustration of random seizure event times over a 60 month (5 year) period, produced by Poisson models with different seizure rates. (A) From top to bottom, the rates illustrated are 1 seizure per: 0.25, 1, 3, 6, or 12 months, respectively. (B) Event times with an underlying rate of 1 seizure / 3 months, up until 36 months (arrow), at which time the rate is set equal to zero (e.g. due to starting a medication). The seizure free period of ~15 months near the beginning of the record is due to chance.
One way to approach this question is to consider a sort of worst case scenario: suppose that, after beginning an intervention, a patient has gone t months without seizures. Assuming pessimistically that this period of seizure freedom is due simply to chance, what is the maximum rate of seizures that could plausibly produce a seizure free period as long as that observed? We can answer this question by solving the Poisson model for the maximum rate, r*, for which the occurrence of a seizure free period of at least t months has at least a 5% probability. We do this by simply setting the expression for the interseizure interval probability distribution equal to 0.05 i.e. setting Pr(Δ t > t∣r*) = e−r*t = 0.05, and then solving for the desired rate. Taking logarithms and rearranging, we obtain r* = ln0.05/t. The logarithm of 0.05 is approximately 3, hence
which is the essential formula underlying the Classical Rule of Three. The principles involved in this derivation are illustrated in Figure 2, which shows the probability of different inter-seizure intervals for various underlying seizure rates. Visual inspection of Figure 2 confirms that the probability of going without seizures decays exponentially as a function of time passed for any given rate of seizures. The seizure-free interval duration at which the probability of having zero seizures decays to 5% is marked by a red line. According to this model, then, a patient with an underlying rate of 1 seizure / month has a 5% probability of going approximately three months without seizures, whereas someone with an underlying rate of 1 seizure / year frequently goes three months or more without seizures, but must go as long as 36 months before reaching this same 5% level of probability. Thus, in either case, the probability of experiencing a seizure free period up to three times longer than the average underlying inter-seizure interval is approximately 5%.
Figure 2.
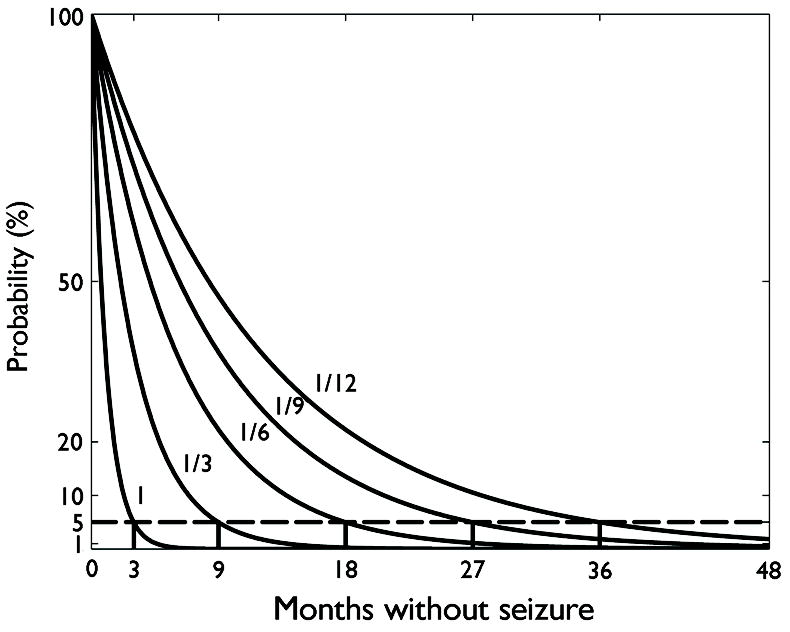
Illustration of the statistical reasoning underlying the Rule of Three as originally formulated. Solid curves show the probability of experiencing a period of seizure freedom (interseizure interval) vs. time, for underlying seizure rates of 1 per 1,3,6,9, or 12 months. The interseizure interval duration for which the probability drops to 5% (dashed line) is marked. As shown, this duration is roughly three times the average interseizure interval.
With these considerations in mind, we are in a position to understand the literal meaning of the Classical Rule of Three. Suppose that, after starting a new medication, a patient has gone for a certain amount of time, t, without seizures. It is useful to state the rule in two different, but equivalent, ways:
To be reasonably confident that a patient’s seizure rate has been reduced, one must wait at least three times longer than the pre-intervention inter-seizure interval.
After t months without seizures, the maximum plausible underlying seizure rate, r*, that is, the maximum average rate of seizures we can reasonably expect in the future, is 3/t.
In other words, when a patient has been seizure free for three times their average pre-medication inter-seizure interval, the most that we can say with confidence is that their seizure frequency has likely been reduced, though perhaps by only a small amount, a statement far short of the declaration that one has become “seizure free”. Stated plainly in these terms, we see that the Classical Rule of Three is unsuitable for evaluating post-intervention seizure freedom. The view expressed in sense (1) is exceedingly conservative, and thus not especially helpful: a patient who previously had monthly seizures and proceeds for 3.1 months after starting a new AED without a single seizure will find little comfort in the idea that it is now reasonable to believe that the underlying seizure rate is probably slightly less than 1 seizure / month. Rather, the patient wants to know to what degree this new data – the fact that a much longer than usual seizure free period has transpired – suggests that seizure freedom will, in fact, persist.
We can also see the unsuitability of the Classical Rule of Three using statement (2). Rather than addressing how long to wait before inferring a reduction in seizure rate, statement (2) can instead be used to address the more directly meaningful question of how long one must wait before the probability of future seizure freedom reaches some acceptably high level of confidence. Let us take the worst case rate cited by the rule, r*, and imagine using this estimate to counsel patients regarding the question: “How long after an intervention must I remain seizure free before the probability of remaining seizure free for at least one year thereafter reaches 95%?” To answer this, we first note that, taking the seizure rate to be r*, the probability of having no seizures in the subsequent 12 months is Pr(N(t) = 0∣r*) = Pr(Δ t > 12∣r*) = e−12r*; therefore, the probability of having one or more seizures in the next 12 months is simply 1 − e−12r*, Table 1 shows these worst case seizure rate estimates according to the Classical Rule of Three – calculated for seizure-free durations of various lengths – together with the consequent probability of having zero seizures in the following year. After six months of seizure freedom, the Classical Rule of Three still allows a ~40% chance of having a seizure in the next year. Obtaining a >95% assurance of remaining seizure free during the next year requires, by this logic, first remaining seizure free for at least 5 years.
Table 1.
Probability of being seizure free over the next year according to the Rule of Three
| Duration of seizure freedom (months) | Estimate of maximum seizure rate (1 seizure / # months) | Probability of no seizure in next year (%) |
|---|---|---|
| 6 | 1 / 2 | 60.7 |
| 12 | 1 / 4 | 77.9 |
| 18 | 1 / 6 | 84.7 |
| 24 | 1 / 8 | 88.3 |
| 36 | 1 / 12 | 92.0 |
| 48 | 1 / 16 | 93.9 |
| 60 | 1 / 20 | 95.1 |
Clearly, the Classical Rule of Three does not lend itself to use as a practical guideline for determination of seizure freedom in any obvious or natural sense. Indeed, in proposing its definition of seizure freedom the ILAE task force (Kwan et al., 2009) specifically points out these limitations of the Classical Rule of Three, and characterizes the proposed definition of seizure freedom as a practical compromise, inspired by but adhering only loosely to the logical of the Classical Rule of Three (words in brackets added for clarification).:
To be 95% certain that a patient’s seizure frequency has at very least decreased (i.e., there has been some therapeutic effect), a seizure-free duration that is at least three times the longest interseizure interval prior to starting a new intervention would need to be observed. It should be noted that, in theory [i.e. according to the logic of the Classical Rule of Three], patients with even more infrequent seizures would have to be followed up for many years to determine whether their seizures had truly come under control. This is not practical, either in research or clinical settings. For this reason we recommend that three times the longest inter-seizure interval be used as an indicator of positive treatment response [i.e. seizure freedom].
Given that patients and physicians are interested in knowing whether an intervention has resulted in seizure freedom rather than just a (probably) decreased rate of seizures, a theoretical framework other than that underlying the Classical Rule of Three is apparently needed. Fortunately, as we will see next, a more practical version of the Classical Rule of Three, consistent with the intended purposes of the definition proposed by the ILAE task force, can be derived from a few reasonable assumptions combined with a principled probabilistic consideration of the problem, using a straightforward application of Bayes’ rule. We shall see that this rule supports the ILAE tasks force’s proposed pragmatic adaptation of the Classical Rule of Three in many important cases of interest, though in other important cases a larger multiple (up to six) of the interseizure interval may be required, hence we call our proposed generalization the “Rule of Three-to-Six”.
Bayesian Formulation of a Rule of Three (to Six) for Seizure Freedom
An essential ingredient missing from the preceding discussion of the Classical Rule of Three is information regarding the a priori probability of seizure freedom conferred by an intervention at the time it is recommended, before any observation period has passed. The omission of this “prior” information is the root cause of the unreasonably long wait times required by the literal interpretation of the Classical Rule of Three. In other words, “letting the data speak for itself”, basing a judgment of seizure freedom solely on the period of observation without seizures, results in longer wait times than clinical experience or clinical epilepsy research suggests is generally necessary. A more principled approach would be to combine statistical information regarding general rates of seizure freedom from published research studies (i.e. pre-test probability of seizure freedom) with the patient-specific data at hand (i.e. time gone without seizures since intervention) to decide when to proclaim seizure freedom.
This needed fusion of probabilistic information is accomplished by means of Bayes’ rule (Jaynes & Bretthorst, 2003; Newman & Kohn, 2009; Westover et al., 2011). In mathematical notation, Bayes’ rule as applied to the case at hand is expressed as
Translated from mathematical notation, this equation can be interpreted as follows. After an intervention (e.g. starting a medication), the probability distribution over the possible seizure rates, r, given that no seizures have occurred so far after t months of observation, is obtained by multiplying two factors together: (1) the “pre-test” or prior probability distribution, Pr (r) (i.e. the distribution of rates, based on known efficacy data, that could have been quoted to the patient at the time of the intervention based on efficacy data), and (2) the likelihood function, which gives the probability of having no seizures over a period of t months when the patient’s underlying rate seizure rate is assumed to be r, Pr(N(t) = 0∣r, t) = Pr (Δ t > t∣r) = e−rt. The additional factor in the denominator, Z, is simply a normalization constant which ensures that the total probability (area under the distribution) is equal to one.
Model #1: All-or-none response distribution
Once we understand how Bayes’ rule can be employed for this application, we must decide how to model the pretest probability of becoming seizure free. For illustration purposes, we will focus first on the case of medication naive adult patients with epilepsy undergoing a first trial of AED therapy. For such patients, epileptologists commonly counsel patients that roughly 50-65% of patients will become seizure free, while the remaining 35-50% will “not respond”, figures based largely on the influential work of Kwan and Brodie (Kwan & Brodie, 2000, 2001). While these studies defined seizure freedom over a limited observational window, it is nevertheless instructive to examine the simple mathematical model that follows if we take these statistics literally. Thus, let us assume simplistically that, a priori, initiating a new AED confers a 65% probability of becoming seizure free, i.e. of effectively setting the patient’s underlying seizure rate to zero, while the remaining 35% of patients experience no change in their underlying seizure rate. In this case, the prior probability distribution Pr(r) is a simple two-bin histogram with values Pr(r = 0) = 65%, and Pr(r = r0) = 35%., where r0 denotes the pre-intervention seizure rate. The challenge then becomes determining into which of these two groups the patient has landed after starting a new medication. Although the distinction cannot immediately be made – since absence of seizures can happen in either case for some time – seizure freedom becomes progressively less likely with the passage of time for “non-responders.” How long must one wait within this framework before the certainty that seizure freedom has been achieved exceeds 95%?
This question can be answered by straightforward application of Bayes’ rule. We wish to calculate the probability of seizure freedom (i.e. that the seizure rate has dropped to zero) as a function of both the duration of seizure freedom since starting a medication, t, and the pre-intervention seizure rate, r0 This is:
Substituting Pr(r = 0) = 0.65, Pr(r = r0) = 0.35, and P(N(t) = 0∣r = 0) = 1, we arrive at:
Finally, let us re-scale the time axis by the patient’s pre-intervention seizure rate by defining τ = r0t, in which case we have simply
This statement demonstrates that the probability of having zero seizures declines with time relative to the duration of the patient’s typical pre-intervention inter-seizure interval. Stated another way, patients with longer inter-seizure intervals prior to AED therapy must wait longer after beginning AEDs to be confident that they have achieved seizure freedom. This probability function is illustrated in Figure 3, from which we see that, under this model, waiting three times the pre-intervention inter-seizure interval is sufficient to provide a greater than 95% confidence of seizure freedom. In fact, as Figure 3 shows, waiting three times the pre-intervention inter-seizure interval still offers 95% probability of future seizure freedom even when the a priori probability of becoming seizure free upon starting a new medication is decreased to 50%. This simplified model thus provides a rough justification for a “Bayesian” Rule of Three.
Figure 3.
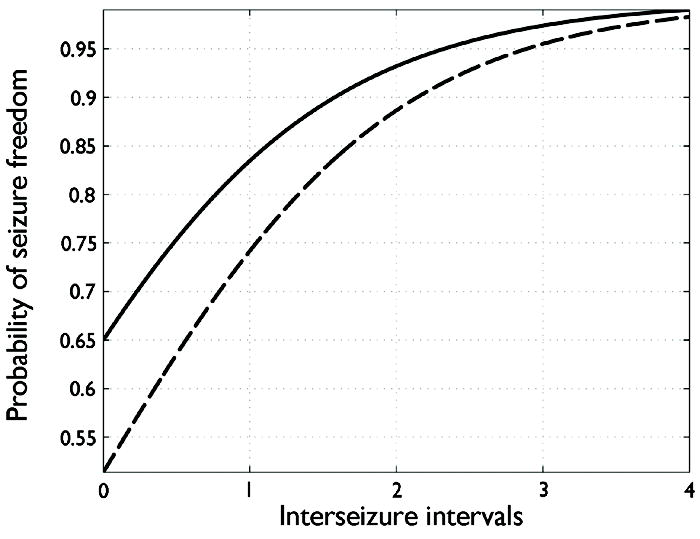
Illustration of Model #1 (see text). Plot of the probability, as a function of time gone without seizures since starting medication, of belonging to the group of “responders” who completely stop having seizures after initiating AED medication. The time axis is given in units of pre-intervention average inter-seizure interval (the reciprocal of the pre-intervention rate). The solid and dashed lines show the probability of seizure freedom assuming an a priori probabilities of seizure freedom of 65% and 50%, respectively. In both cases, waiting three times the pre-intervention interseizure interval is sufficient to achieve >95% confidence of seizure freedom.
It is natural to ask how the determination of seizure freedom must be modified under this simple model when the a priori probability of attaining seizure freedom from an intervention falls well below the 50-65% range considered above. For example, many patients encountered in epilepsy specialty clinics have already “failed” one or more previous AEDs. In these cases, the odds of achieving seizure freedom from additional AED trials is generally estimated at 5-10% (Kwan & Brodie, 2000). Similarly, patients with nonlesional or multifocal epilepsy considering epilepsy surgery typically face odds considerably worse than the oft-cited up-to-80% probability of seizure freedom for favorably-selected lesional cases (McIntosh et al, 2001). While many of patients can indeed ultimately be declared seizure free after these interventions, given the less favorable initial odds we expect that a longer period of observation will be necessary. This intuition can be made precise within the modeling framework above by substituting into the last formula a generic pre-test probability, α, and solving for the number of pre-intervention interseizure intervals that must pass before reaching 95% probability of seizure freedom, i.e. by solving the following for the value of τ:
The resulting relationship between the pretest probability α and the required observation period τ(α) is plotted in Figure 4. From this plot we see that when the pre-intervention probability of seizure freedom is 5-10%, the required waiting time ranges from roughly 5 to slightly less than 6 times the average pre-intervention interseizure interval.
Figure 4.
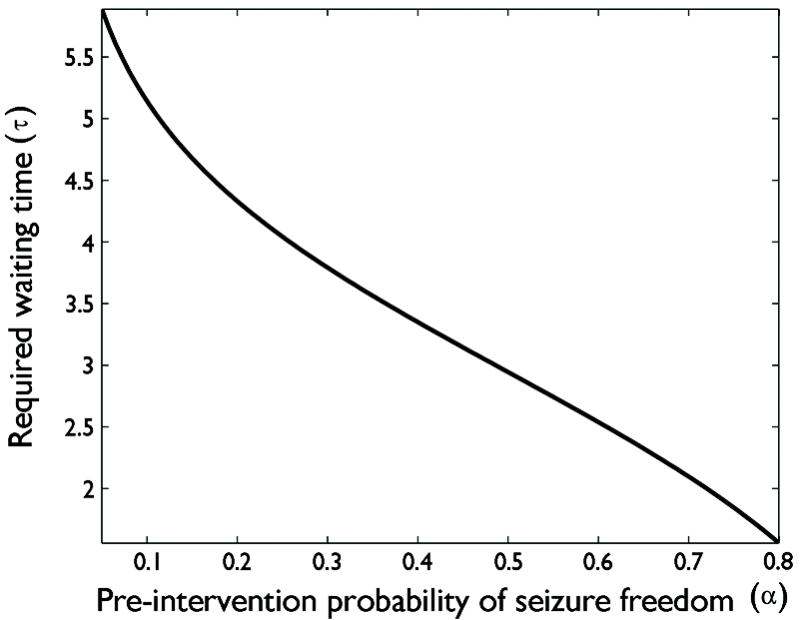
Relationship between the a priori probability that an intervention will render a patient seizure free, α (horizontal axis), and the required seizure-free observation period before one the probability of future seizure freedom reaches 95%, according to Model #1 (see main text). The required waiting time is expressed in units equal to the mean pre-intervention interseizure interval. When the prior probability of seizure freedom is greater than 50%, 3 interseizure intervals is sufficient, and in fact for α = 80%, waiting approximately 1.5 intervals will theoretically suffice. By contrast, when the prior probability of success is less than 50%, a larger number of observation intervals is required, reaching roughly 6 intervals when α drops to 5%.
Hence, the rule governing the required waiting time before declaring seizure freedom, which we may call the “Rule of Three-To-Six”, can be stated as follows: When the a priori probability that an intervention will confer seizure freedom is >50%, then an observation period 3 times the typical pre-intervention interseizure interval is sufficient to achieve greater than 95% certainty that the patient will remain seizure free, whereas when the a priori probability of success is less, a longer waiting time (up to 6 times the typical pre-intervention interseizure interval when the prior probability of success drops to 5%) is required.
Model #2: Continuous but bimodal response distribution
While the model just considered is unrealistic in its binary division of patients into either completely seizure free “responders” or complete “non-responders” experiencing no change in seizure activity whatsoever, qualitatively more realistic versions of the same basic model can yield generally similar conclusions, thus supporting the basic idea of the Rule of Three-To-Six. We again focus for illustration on the case of determining seizure freedom in adult epilepsy patients who have not failed previous therapies. An example of a more realistic modified model is illustrated in Figure 5, where the prior probability Pr(r) is represented not as a binary histogram, but rather as a continuous distribution. The distribution has two modes: (1) one mode that is centered around the pre-intervention seizure rate with some spread around the mean value to represent uncertainty regarding the precise seizure rate as well as the possibility that seizure rates in “non-responders” may change slightly after treatment; (2) and another mode that is concentrated near a seizure rate of zero but also includes some spread to account for those patients who “significantly improve” but nevertheless continue to have seizures at a nonzero rate. For simplicity, we do not attempt to model the fact that in some patients seizure freedom may require additional, adjunctive medications—these may be considered together as a single intervention in the present model. In Figure 4, the “responder” mode was chosen such that 65% of the total probability lies beneath it – representing the ≈65% of patients who respond to AED therapy – while the remaining probability mass lies beneath the second mode – representing the ≈35% of patients who do not respond to AED therapy.
Figure 5.
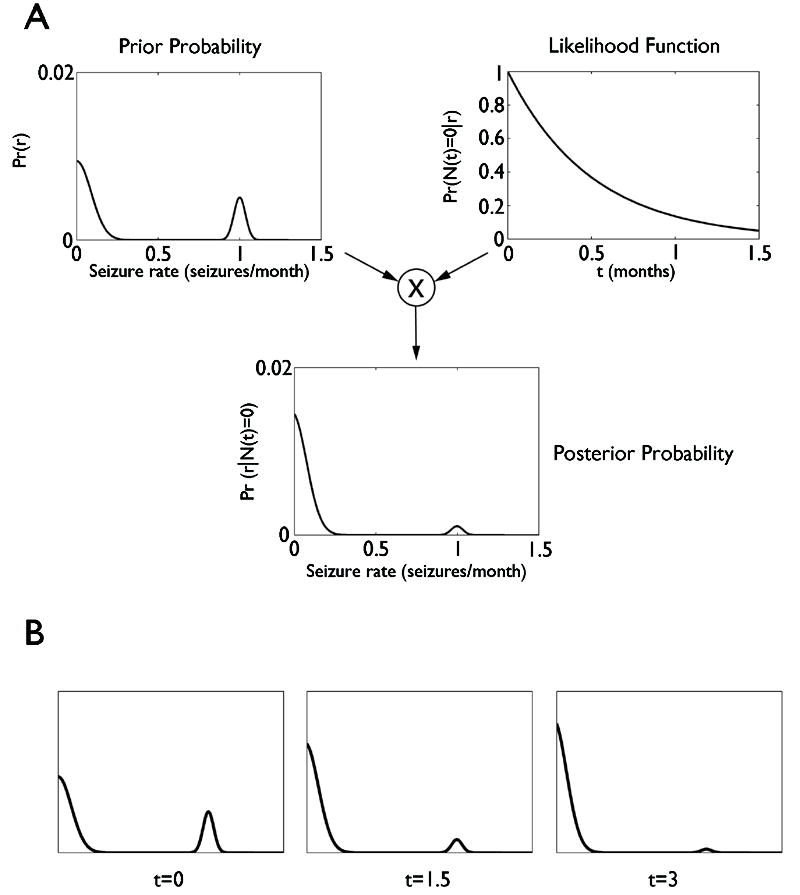
Illustration for “Model #2: Continuous but bimodal response distribution”. Illustration for “Model #2: Continuous but bimodal response distribution”. A: The top left panel shows the expected long-term population-level distribution of seizure rates among patients whose initial seizure rates are clustered around 1 seizure / month. This distribution serves as the prior probability distribution over post-intervention seizure rates, P(r). To apply Bayes’ rule, this distribution is multiplied by the likelihood function, Pr (N(t) = 0∣r), shown in the upper right hand panel for seizure-free observation period of t = 2 months, and a re-normalization of the distribution to ensure a total probability mass equal to one, resulting in the post-observation probability distribution Pr (r ∣ N(t) = 0), shown in the bottom panel in A. As shown, this multiplication and renormalization results in a very significant “damping down” of the probability of remaining in the ‘nonresponder’ group. B: Illustration of how the post-observation probability distribution over seizure rates evolves over time. Three discrete time points are shown, equal to 1, 2, and 3 months.
We combine this model of the pre-test probability, Pr(r), with the Poisson seizure rate model, Pr(N(t) = 0∣r) = e−rt, via Bayes’ rule, i.e. by multiplying these together and then normalizing (dividing by the total area under the curve to ensure that the total probability is equal to 1). The result is illustrated in Figure 5B for a patient with a pre-intervention seizure rate of 1 seizure / month, after a seizure free observation period lasting 2 months. We see that multiplying by the exponentially decaying curve (the likelihood function) has the effect of dampening the probability that the patient belongs to the “non-responder” group and amplifying the possibility that the patient is a “responder.” The effect of continued observation over time is illustrated in Figure 5C. We see that, qualitatively, after being seizure free for approximately three times the patient’s pre-intervention inter-seizure interval, the probability of belonging to the non-responder group drops nearly to zero, again in agreement with the general operational concept of the ILAE task force’s proposed pragmatic adaptation of the Classical Rule of Three, though justified on different grounds than those from which the Classical Rule of Three was originally derived. Unsurprisingly, for cases in which the pre-intervention probability of success is lower, similar calculation show that, as above in analogous analysis of the simpler Model #1, longer waiting times are required.
Discussion
The question of how to determine what constitutes an adequate period without seizures before a patient can be presumed seizure free after an intervention is frequently encountered in clinical practice and has important implications not only for patient management (e.g. when should patients feel secure returning to high-risk work environments?), but also potentially for public policy (e.g. when can patients safely be allowed to drive?). Current laws require a patient to be seizure free anywhere from three months to one year before they can legally drive, irrespective of the patient’s underlying seizure rate. Specifying an arbitrary duration of seizure freedom without considering the variation in seizure rates or a priori probability of an intervention’s success rate may require some patients to wait longer than necessary before driving and allow others to return to driving before it is safe to do so. Many physicians counsel newly diagnosed patients with epilepsy based on data regarding a priori probabilities of rates of seizure remission, e.g. the “rule” that, a priori, approximately 50-65% of newly diagnosed, medication-naive adult epilepsy patients become seizure free upon starting medication while 35-50% of remain refractory. The corresponding probabilities for patients who have failed 1-2 previous medications are closer to 5-10% (Kwan & Brodie, 2000, 2001); for surgery the probability of success reaches as high as 80% to much lower depending on identifiable risk factors (McIntosh et al, 2001).
Beyond these figures, some guidance regarding how long to wait before identifying a patient as belonging to the “seizure free” outcome group comes from the recent recommendation of the ILAE task force, which proposes to declare that seizure freedom has been achieved in when there is “freedom from all types of seizures for 12 months or three times the pre-intervention inter-seizure interval, whichever is longer” (Kwan et al., 2009). This definition of seizure freedom represents a practical adaptation of rather than a rigorous consequence of the statistical considerations underlying Classical Rule of Three as formulated in the classic 1983 paper by Hanley and Lippman-Hand (Hanley & Lippman-Hand, 1983; Jovanovic & Levy, 1997), strict application of which may require waiting much longer than the recommended interval before patients can be reasonably declared “seizure free”.
Nevertheless, we have also seen how a rule in the spirit of that proposed by the ILAE task force can be placed on a solid theoretical foundation using straightforward considerations from probability theory. More specifically, we have shown how Bayes’ rule can be used to combine data regarding the a priori probability of responding to an intervention with patient-specific data regarding how long a patient has remained seizure free so far after an intervention to estimate the probability that the patient will remain seizure free thereafter. In its simplest form, explored in Model #1, this probabilistic framework suggests adopting a modification of the rule proposed by the ILAE task force, which we call the “Rule of Three-To-Six”: Future seizure freedom can be inferred after an observation period of three times the average pre-intervention interseizure interval so long as the pre-intervention probability of success exceeds 50%, whereas up to six times may be required when the pre-intervention probability of success drops as low as 5%.
As briefly explored in Model #2, by modeling the relevant a priori intervention response probabilities, the Bayesian approach used in this paper can also be adapted to guide determination of response to medication conceptualized in more complex ways (e.g. a “meaningful improvement”, such as >50% reduction of seizure frequency rather than a strictly complete cessation of seizures).
It should be borne in mind that the models explored here contain simplifying assumptions that may not be applicable in certain cases. First, our model treats seizure recurrences as random events. This is a reasonable way to model seizure occurrences where the underlying provocative factors are unknown (i.e. for seizures which seem to occur “out of the clear blue”), or unpredictable (e.g. minor illnesses or other unforeseeable stressors), but probably does not appropriately model systematic effects under a patient’s control, such as medication non-compliance or unfavorable lifestyle choices. Second, some patients with epilepsy exhibit either strongly non-random tendencies, as in epilepsy which varies catamenially. Such patterns violate the basic assumption of our analysis that seizure activity follows a constant-rate (Poisson) random process model. Third, between the random and strictly predictable cases lie cases in which seizures show a greater or lesser degree of clustering. In cases where prominent temporal clustering occurs, our analysis may still be reasonably applied if all seizures within a distinct cluster are considered as a single “event”. In cases of weaker clustering, where the boundaries between clusters are indistinct, the basic analysis may still be reasonably applied, although with minor modifications. More specifically, intermediate levels of clustering lead to modest increases in the period of observation required by the Rule of Three-To-Six (see Supplemental Material). Fourth, the appropriate a priori probability of seizure freedom, an essential ingredient in the Rule of Three-To-Six, can be only loosely approximated from the current literature surrounding anti-epileptic drug efficacy, which often treats response to anti-seizure interventions as an “all or none” phenomenon. The development of richer, more patient-specific methods for predicting response to epilepsy interventions thus remains an area where further research is needed.
These limitations notwithstanding, to the extent that – as pointed out by the ILAE task force in quoting Voltaire – “the perfect is the enemy of the good”, the Rule of Three-To-Six proposed herein provides reasonable practical guidance for evaluating seizure freedom in response to pharmacological, surgical, and other interventions.
Supplementary Material
Acknowledgments
SSC, AJC, and MBW receive support from NINDS NS062092.
Footnotes
Disclosure of Conflicts of Interest None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
References
- Balish M, Albert PS, Theodore WH. Seizure frequency in intractable partial epilepsy: a statistical analysis. Epilepsia. 1991;32:642–649. doi: 10.1111/j.1528-1157.1991.tb04703.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol. 2010;9:27–29. doi: 10.1016/S1474-4422(09)70304-7. [DOI] [PubMed] [Google Scholar]
- Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA. 1983;249:1743–1745. [PubMed] [Google Scholar]
- Haut SR, Lipton RB, LeValley AJ, Hall CB, Shinnar S. Identifying seizure clusters in patients with epilepsy. Neurology. 2005;65:1313–1315. doi: 10.1212/01.wnl.0000180685.84547.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes ET, Bretthorst GL. Probability theory: the logic of science. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Jovanovic BD, Levy PS. A Look at the Rule of Three. The American Statistician. 1997;51:137–139. [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia. 2001;42:1255–1260. doi: 10.1046/j.1528-1157.2001.04501.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2009;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Wilson SJ, Berkovic SF. Seizure Outcome after Temporal Lobectomy: Current Research Practice and Findings. Epilepsia. 2001;42:1288–1307. doi: 10.1046/j.1528-1157.2001.02001.x. [DOI] [PubMed] [Google Scholar]
- Newman TB, Kohn MA. Evidence-based diagnosis. Cambridge University Press; Cambridge: 2009. [Google Scholar]
- Papoulis A. Probability, random variables, and stochastic processes. 2. (McGraw-Hill series in electrical engineering. Communications and information theory): McGraw-Hill; New York: 1984. [Google Scholar]
- Westover MB, Westover KD, Bianchi MT. Significance testing as perverse probabilistic reasoning. BMC Medicine. 2011;9:20. doi: 10.1186/1741-7015-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


