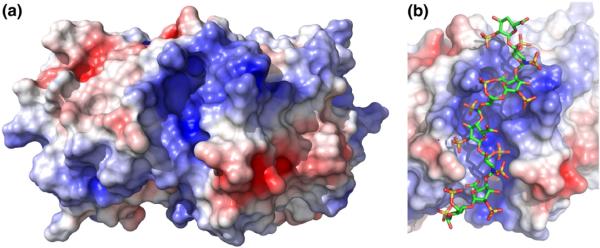
Fig. 7.
The positively charged cleft on the PKR kinase domain. (a) Electrostatic surface of the kinase domain. A solvent-accessible surface of the PKR kinase domain alone was generated from the crystal structure of a complex of the kinase domain and eIF2α (Protein Data Bank ID: 2A1A) using PyMOL. The dimer interface is located on the top surface of the kinase domain monomer. The electrostatic surface was produced using APBS and was contoured at ±5 kT. (b) Model of dp8 bound to the kinase domain in the positively charged cleft. The dp8 ligand was docked using AutoDock Vina. See Materials and Methods for details.