Abstract
Purpose
To evaluate the accuracy of preoperative keratometers used in cataract surgery with toric intraocular lens (IOL).
Methods
Twenty-five eyes received an AcrySof toric IOL implantation. Four different keratometric methods, a manual keratometer, an IOL master, a Pentacam and an auto keratometer, were performed preoperatively in order to evaluate preexisting corneal astigmatism. Differences between the true residual astigmatism and the anticipated residual astigmatism (keratometric error) were compared at one and three months after surgery by using a separate vector analysis to identify the keratometric method that provided the highest accuracy for astigmatism control.
Results
The mean keratomeric error was 0.52 diopters (0.17-1.17) for the manual keratometer, 0.62 (0-1.31) for the IOL master, 0.69 (0.08-1.92) for the Pentacam, and 0.59 (0.08-0.94) for the auto keratometer. The manual keratometer was the most accurate, although there was no significant difference between the keratometers (p > 0.05). All of the keratometers achieved an average keratometric error of less than one diopter.
Conclusions
Manual keratometry was the most accurate of the four methods evaluated, although the other techniques were equally satisfactory in determining corneal astigmatism.
Keywords: Astigmatism, Cataract, Keratometer, Keratometric error, Toric intraocular lenses
A significant number of patients who undergo cataract surgery have a varying degree of preexisting corneal astigmatism. An estimated 15% to 29% of patients with cataracts have more than 1.50 diopters (D) of preexisting astigmatism [1,2], and approximately 2% of all cataract patients have astigmatism of more than 4.00 D [1].
Astigmatism can be reduced or eliminated with several techniques, which include selective positioning of the phacoemulsification incision, corneal relaxing incisions, limbal relaxing incisions, excimer laser keratectomy and toric intraocular lens (IOL) implantation. Several reports have shown that toric IOL implantation during cataract surgery is an effective and safe method to reduce corneal astigmatism [3-9].
The optical effect that results from toric IOL implantation depends on the accurate measurement of the preoperative corneal astigmatism. Inaccurate measurements may result in failure to reduce the astigmatism or it may even result in worsened corneal astigmatism. Alcon, the manufacturer of the AcrySof toric IOLs, recommends the use of a manual keratometer to measure preoperative corneal astigmatism, but no studies have actually compared the accuracy of the various astigmatism-measuring instruments. The purpose of this study was to evaluate the accuracy of the various keratometers that are used to make preoperative measurements prior to cataract surgery with toric IOL.
Materials and Methods
This prospective clinical study included 25 eyes from 23 patients who had received AcrySof toric IOL implantation between the dates of April 2008 to April 2009. Inclusion criteria were the presence of cataracts, less than 80 years of age, having a preoperative regular corneal astigmatism greater than 1.50 D, and a normal macular finding. Exclusion criteria were having an irregular corneal astigmatism, a regular astigmatism greater than 5.00 D, tear-film abnormalities, or extensive macular disease. Informed consent was obtained from all of the patients after the nature and possible consequences of both the study and the surgery were fully explained. Patients received a complete preoperative ophthalmic examination, including slit lamp examination, IOP measurement using Goldmann applanation tonometry, preoperative manifest refraction, keratometry, and fundus examination.
Preoperative corneal astigmatism was evaluated by a single trained examiner using four different keratometers: a SO-21 manual keratometer (Shin-Nippon, Tokyo, Japan), a 420 auto keratometer (Allergan Humphrey, San Leandro, CA, USA), a Pantacam (Oculus, Wetzlar, Germany), and an IOL master (Zeiss, Jena, Germany). Intra-grader repeatability was evaluated and the coefficient of repeatability (COR) for the mean keratometric power was calculated. Axial length was measured with the IOL master and the Humphrey A-scan. Calculation of the IOL axis placement was performed using a toric IOL calculator program (http://www.acrysoftoriccalculator.com). Preoperative keratometry, biometry data, incision location, and the surgeon-estimated surgically-induced corneal astigmatism were used to determine the appropriate AcrySof toric IOL model, spherical equivalent lens power, and axis of placement in the eye. The SRK/T formula was used for spherical IOL power calculation. The targeted refraction was emmetropia.
All of the surgeries were performed by the same surgeon using topical anesthesia. With the patient seated at the slit lamp and with a coaxial thin slit adjusted to the 0- to 180-degree axis, the corneal limbus was marked at the 0- and 180-degree positions with a sterile marker after vertical alignment with the patient's head. Next, with the patient lying on the surgical table, the steep corneal meridian was identified and marked using a Marquez gauge with the aid of the preplaced reference points. Phacoemulsification was performed through a 2.75 mm temporal corneal incision. After phacoemulsification, a foldable AcrySof toric IOL (AcrySof SA60AT; Alcon Laboratories, Fort Worth, TX, USA) was inserted into the capsular bag using a Monarch II injector (Alcon Laboratories), which was then rotated approximately 15 degrees off-axis before the ophthalmic viscosurgical device (OVD; sodium hyaluronate 1%, Provisc) was removed. After the OVD removal, the IOL was rotated to the final position by aligning the toric reference marks. A corneal suture was then made that was scheduled to be removed one week after surgery. Postoperative examinations were performed one day, one week, and one and three months after surgery. The manifest refraction (MR) and keratometric value were measured at the one month follow-up appointment, and all of the patients had a complete postoperative ophthalmic examination. Toric IOL rotation was measured using the slit lamp in one-degree steps through pupils that were dilated with tropicamide. A thin coaxial slit was projected in front of the eye and rotated until the thin slit projection overlapped with the axis marks of the IOL.
MR was performed in order to evaluate residual astigmatism. The residual corneal astigmatism that was based on the MR measurement was compared to the anticipated residual astigmatism, which is calculated using an online program. We defined the keratometric error (KE), as follows: KE = (actual postoperative astigmatism - anticipated residual astigmatism) / toricity of implanted IOL. We calculated KE using the vector calculator program VECTrAK version 1.5. An example of how the KE was calculated is as follows: preoperative corneal astigmatism as measured by manual keratometer (OD): 2.20 × 99°; surgically-induced astigmatism: 0.50 × 90°; crossed cylinder result (corneal plane): 2.68 × 97°; AcrySof Toric IOL: SN60T5 (cylinder power at corneal plane 2.06 D); axis of IOL placement: 97°; anticipated residual astigmatism: 0.62 × 97°; IOL rotation one month after surgery: 3°; loss of toric IOL effect to correct astigmatism: 10 percent of the toricity; corrected anticipated residual astigmatism: 2.68 - (2.06 × 0.9) = 0.82 × 97°; actual residual astigmatism (MR at one-month after surgery): 1.25 × 90°; difference between the two values of astigmatism: 0.50 × 78° (by vector calculator); KE = 0.50 / 2.06 = 0.24
Statistical analysis was performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). One way ANOVA and Tukey's b-test were used for performing comparative statistics. A p-value less than 0.05 were considered significant in our analyses.
Results
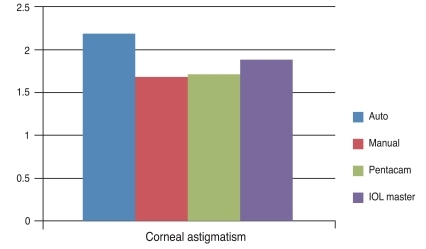
The COR of the mean keratometric power was ±0.21 D for the auto keratometer, ±0.20 D for the manual keratometer, ±0.32 D for the Pentacam, and ±0.22 D for the IOL master. The demographic and clinical characteristics of the patients are shown in Table 1. The average preoperative corneal astigmatism was 2.18 ± 0.67 D by the auto keratometer, 1.68 ± 0.55 D by the manual keratometer, 1.74 ± 0.63 D by the Pentacam, and 1.90 ± 0.63 D by the IOL master. Average astigmatism as measured by the auto keratometer appeared to be higher than what was measured with the other instruments, but this difference was not significantly different (p = 0.06) (Fig. 1).
Table 1.
Patient demographics and characteristics
SD = standard deviation; OD = right eye; OS = left eye; OU = both eyes; UCVA = uncorrected visual acuity; logMAR = logarithm of the minimum angle of resolution; BCVA = best corrected visual acuity; D = diopter; IOL = intraocular lens.
Fig. 1.
Comparison of the preoperative corneal astigmatism as measured by several keratometers. No significant differences were seen between the four groups (p = 0.06). IOL = intraocular lens.
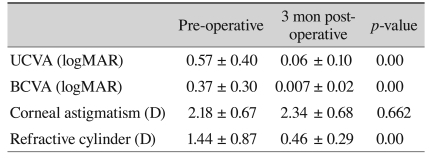
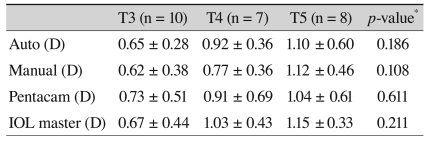
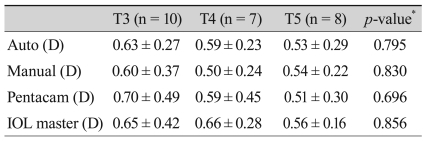
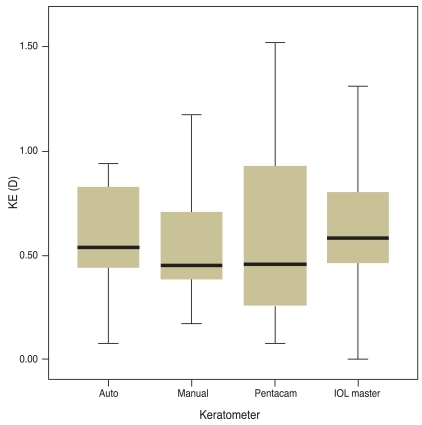
Postoperative results are shown in Table 2. The differences between postoperative residual corneal astigmatism and the anticipated residual astigmatism are listed in Table 3. T5 IOL showed the largest differences, which were then followed by T4 and T3, although these were not significant, and to reduce the confounding factors we divided the differences by the toricities of the implanted IOL. Comparison KE as toricity showed no significant differences (Table 4). The average KE was 0.59 D (0.08-0.94) by the auto keratometer, 0.52 D (0.17-1.17) by the manual keratometer, 0.61 D (0.08-1.52) by the Pentacam, and 0.62 D (0-1.31) by the IOL master. The median KE value was 0.54 D by the auto keratometer, 0.45 D by the manual keratometer, 0.46 D by the Pentacam, and 0.58 D by the IOL master (Fig. 2). Although the manual keratometer had both the lowest average and median KE values measured, the differences were not significant between the four methods studied. All of the keratometers achieved an average KE value less than 1 D.
Table 2.
Visual acuity and manifest refraction before and three months after AcrySof toric IOL implantation
UCVA = uncorrected visual acuity; logMAR = logarithm of the minimum angle of resolution; BCVA = best corrected visual acuity; D = diopter.
Table 3.
Comparison of the differences between the residual corneal astigmatism and the anticipated residual astigmatism
Values are presented as mean ± SD.
D = diopter; SD = standard deviation; IOL = intraocular lens.
*One way ANOVA.
Table 4.
Comparison of the KE with regard to toricity
Values are presented as mean ± SD.
KE = (actual postoperative astigmatism - anticipated residual astigmatism) / toricity of implanted IOL.
KE = keratometric error; SD = standard deviation; D = diopter; IOL = intraocular lens.
*ANOVA.
Fig. 2.
Comparison of the keratometric error of several keratometers. No significant differences were observed between the four groups (p = 0.944). KE = keratometric error; D = diopter; IOL = intraocular lens.
Discussion
Preoperative keratometric data must be accurate in order to reduce astigmatism effectively when using a toric IOL. The reliability of the keratometry depends on the repeatability, reproducibility and on the validity of the keratometry measurements. In our study, the COR of the mean keratometric power was ±0.21 D for the auto keratometer, ±0.20 D for the manual keratometer, ±0.32 D for the Pentacam, and ±0.22 D for the IOL master. All of these methods showed good reproducibility.
There have been many reports that have tried to prove the reliability of the auto keratometer. Davies et al. [10] have reported that the refractive error as measured by the Shin-Nippon NVision-K 5001 autorefractor was similar to that of subjective refraction and keratometry as measured by the Javal-Schiotz technique, for both the horizontal and vertical meridians, and that the autorefractor was found to be accurate and provided reproducible data. Gonzalez-Meijome et al. [11] have reported that the central corneal curvature data obtained by an ARK 700A auto keratometer (Nidek, Gamagori, Japan) and a Medmont E300 corneal topographer (Medmont Pty Ltd., Melbourne, Australia) were similar to one another. Sheppard and Davies [12] have demonstrated that the Grand Seiko auto keratometer is very similar (p = 0.77) to subjective refraction, and report that the instrument was both accurate and reliable. While no reports were found on the auto keratometer that was used in this study, we observed that other auto keratometers had good reproducibility.
Shankar et al. [13] have found that the corneal curvature as measured by a Pentacam showed good reproducibility, both anteriorly (mean COR, ±0.28 D) and posteriorly (COR, ±0.11 D). In our study, the COR for mean keratometric power as measured by the Pentacam was ±0.32 D, which is comparable to the results reported by Shankar et al. [13].
It has been well demonstrated that the IOL Master is highly precise, accurate, and reproducible [14-19]. Kim et al. [20] have demonstrated that the IOL Master has fairly good results in refractive prediction for cataract surgery. However, there have only been a few reports that have focused on keratometric accuracy. Instead, many of the previous reports have only focused on the efficacy of toric intraocular lens, but the IOL Master was often used to evaluate the preoperative keratometry [5,21,22], which may have indirectly validate that the IOL Master is both reliable and accurate.
However, our ability to evaluate repeatability was limited because we evaluated only the COR of the mean keratometric power. To evaluate the reproducibility of the corneal astigmatism measurements, not only should the degree of astigmatism be measured but also the axis of the astigmatism should be simultaneously evaluated by using vector analysis, or at least a separate evaluation of the reproducibility of both factors should be conducted.
We introduced KE to evaluate the accuracies of the keratometers that were used in this study. KE was defined as the difference between the actual residual astigmatism and the anticipated residual astigmatism divided by the toricity of the implant. We hypothesized that this would provide the greatest accuracy in astigmatism control in order to determine which keratometer was most accurate and to also determine the degree of error for each of the instruments. The difference between the two astigmatic values was then divided by the toricity in order to allow for comparisons of KE across different toricities.
This formula had its limitations, since the postoperative astigmatism can be influenced by not only the IOL toricity, but also by the IOL rotation, which is not considered in the formula we used. We also assumed that the astigmatism was not induced by the IOL itself.
In spite of these limitations, the comparison of KEs from different keratometers was meaningful since the four keratometers were evaluated under the same environment and conditions. This was the first study to evaluate the reliability of keratometers in correcting astigmatism with toric IOL. The results show that the manual keratometer was the most accurate of the ones tested, but the differences between the instruments were not significant. Bauer et al. has reported that manual keratometry (Javal), automated keratometry by optical biometry (IOL Master), and corneal topography all gave comparable results in regards to measuring corneal astigmatism [5]. This is consistent with our findings, with the exception that Bauer only compared preoperative astigmatic values. The manual keratometry was mandatory practice in the AcrySof clinical trial [23], and the three other methods were found to be equally competent at determining corneal astigmatism.
In conclusion, manual keratometry was found to be the most accurate in this study, although the other methods that were studied were equally suitable for determining corneal astigmatism for implanting toric IOL. We suggest that a study should be done to validate our findings by having longer follow-up times and a larger number of samples.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hoffer KJ. Biometry of 7,500 cataractous eyes. Am J Ophthalmol. 1980;90:360–368. doi: 10.1016/s0002-9394(14)74917-7. [DOI] [PubMed] [Google Scholar]
- 2.Ninn-Pedersen K, Stenevi U, Ehinger B. Cataract patients in a defined Swedish population 1986-1990. II. Preoperative observations. Acta Ophthalmol (Copenh) 1994;72:10–15. doi: 10.1111/j.1755-3768.1994.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 3.Mendicute J, Irigoyen C, Aramberri J, et al. Foldable toric intraocular lens for astigmatism correction in cataract patients. J Cataract Refract Surg. 2008;34:601–607. doi: 10.1016/j.jcrs.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Weinand F, Jung A, Stein A, et al. Rotational stability of a single-piece hydrophobic acrylic intraocular lens: new method for high-precision rotation control. J Cataract Refract Surg. 2007;33:800–803. doi: 10.1016/j.jcrs.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Bauer NJ, de Vries NE, Webers CA, et al. Astigmatism management in cataract surgery with the AcrySof toric intraocular lens. J Cataract Refract Surg. 2008;34:1483–1488. doi: 10.1016/j.jcrs.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Horn JD. Status of toric intraocular lenses. Curr Opin Ophthalmol. 2007;18:58–61. doi: 10.1097/ICU.0b013e328011f9bf. [DOI] [PubMed] [Google Scholar]
- 7.Dardzhikova A, Shah CR, Gimbel HV. Early experience with the AcrySof toric IOL for the correction of astigmatism in cataract surgery. Can J Ophthalmol. 2009;44:269–273. doi: 10.3129/i09-048. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Mesa R, Carrasco-Sanchez D, Diaz-Alvarez SB, et al. Refractive lens exchange with foldable toric intraocular lens. Am J Ophthalmol. 2009;147:990–996. 996.e1. doi: 10.1016/j.ajo.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Mendicute J, Irigoyen C, Ruiz M, et al. Toric intraocular lens versus opposite clear corneal incisions to correct astigmatism in eyes having cataract surgery. J Cataract Refract Surg. 2009;35:451–458. doi: 10.1016/j.jcrs.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 10.Davies LN, Mallen EA, Wolffsohn JS, Gilmartin B. Clinical evaluation of the Shin-Nippon NVision-K 5001/Grand Seiko WR-5100K autorefractor. Optom Vis Sci. 2003;80:320–324. doi: 10.1097/00006324-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Meijome JM, Jorge J, Queiros A, et al. A comparison of the ARK-700A autokeratometer and Medmont E300 corneal topographer when measuring peripheral corneal curvature. Ophthalmic Physiol Opt. 2004;24:391–398. doi: 10.1111/j.1475-1313.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 12.Sheppard AL, Davies LN. Clinical evaluation of the Grand Seiko Auto Ref/Keratometer WAM-5500. Ophthalmic Physiol Opt. 2010;30:143–151. doi: 10.1111/j.1475-1313.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 13.Shankar H, Taranath D, Santhirathelagan CT, Pesudovs K. Anterior segment biometry with the Pentacam: comprehensive assessment of repeatability of automated measurements. J Cataract Refract Surg. 2008;34:103–113. doi: 10.1016/j.jcrs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Connors R, 3rd, Boseman P, 3rd, Olson RJ. Accuracy and reproducibility of biometry using partial coherence interferometry. J Cataract Refract Surg. 2002;28:235–238. doi: 10.1016/s0886-3350(01)01179-8. [DOI] [PubMed] [Google Scholar]
- 15.Choi JH, Roh GH. The reproducibility and accuracy of biometry parameter measurement from IOL Master(R) J Korean Ophthalmol Soc. 2004;45:1665–1673. [Google Scholar]
- 16.Vogel A, Dick HB, Krummenauer F. Reproducibility of optical biometry using partial coherence interferometry: intraobserver and interobserver reliability. J Cataract Refract Surg. 2001;27:1961–1968. doi: 10.1016/s0886-3350(01)01214-7. [DOI] [PubMed] [Google Scholar]
- 17.Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–773. doi: 10.1007/s004170000188. [DOI] [PubMed] [Google Scholar]
- 18.Goyal R, North RV, Morgan JE. Comparison of laser interferometry and ultrasound A-scan in the measurement of axial length. Acta Ophthalmol Scand. 2003;81:331–335. doi: 10.1034/j.1600-0420.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Kim HJ, Joo CK. Comparison of IOL Master, A-scan and Orbscan II for measurement of axial length and anterior chamber depth. J Korean Ophthalmol Soc. 2003;44:1519–1527. [Google Scholar]
- 20.Kim SM, Choi J, Choi S. Refractive predictability of partial coherence interferometry and factors that can affect it. Korean J Ophthalmol. 2009;23:6–12. doi: 10.3341/kjo.2009.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed II, Rocha G, Slomovic AR, et al. Visual function and patient experience after bilateral implantation of toric intraocular lenses. J Cataract Refract Surg. 2010;36:609–616. doi: 10.1016/j.jcrs.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Statham M, Apel A, Stephensen D. Comparison of the AcrySof SA60 spherical intraocular lens and the AcrySof Toric SN60T3 intraocular lens outcomes in patients with low amounts of corneal astigmatism. Clin Experiment Ophthalmol. 2009;37:775–779. doi: 10.1111/j.1442-9071.2009.02154.x. [DOI] [PubMed] [Google Scholar]
- 23.Husain SE, Kohnen T, Maturi R, et al. Computerized videokeratography and keratometry in determining intraocular lens calculations. J Cataract Refract Surg. 1996;22:362–366. doi: 10.1016/s0886-3350(96)80251-3. [DOI] [PubMed] [Google Scholar]