Abstract
New direct and indirect acting factor Xa (FXa) and thrombin inhibitors are being developed to overcome the downsides of the conventional anticoagulants – unfractionated and low molecular weight heparins and vitamin K antagonists. Ximelagatran and idraparinux failed to demonstrate an acceptable safety profile. Rivaroxaban and dabigatran are approved for the post-operative prevention of thromboembolic complications after elective hip or knee replacement surgery; dabigatran is approved for the prevention of embolism in patients with atrial fibrillation in an increasing number of countries. Several novel indirect antithrombin-dependent anticoagulants as well as antithrombin-independent oral direct FXa and thrombin inhibitors are investigated in multiple indications for the prophylaxis and treatment of venous thromboembolism and the prophylaxis of arterial thrombotic disorders. Quality-adjusted life years costs and incremental cost-effectiveness ratios are relatively high at present, but may decrease after approval of more new anticoagulants for additional indications.
Keywords: anticoagulants, factor Xa inhibitors, thrombin inhibitors, atrial fibrillation
Introduction
Immediately acting unfractionated heparins (UFHs), low molecular weight heparins (LMWHs), hirudins or argatroban (Hirsh et al., 2008), and slowly acting vitamin K antagonists (VKAs) (Ansell et al., 2008) reduce the morbidity and mortality of patients at risk of recurrent venous thromboembolism (VTE) (Kearon et al., 2008), cerebral and non-cerebral embolism (Singer et al., 2008), and coronary occlusion or reocclusion (Becker et al., 2008). The use of these anticoagulants is limited by several drawbacks. Heparins, LMWHs, hirudins and argatroban have to be administered intravenously or subcutaneously and require dose adjustment guided by monitoring of the anticoagulant effect. LMWHs have to be administered subcutaneously, with the dose being adjusted in older patients and in renal impairment. Severe side effects such as heparin-induced thrombocytopenia or the generation of antibodies to hirudins and other drug-related side effects limit their administration (Warkentin et al., 2008). The main downsides of VKAs are the requirement of regular dose adjustments by monitoring the anticoagulant effect, the low prevalence of international normalized ratio (INR) values within the therapeutic range (2–3) (Baker et al., 2009), the interactions with food and many drugs, severe intracranial and extracranial bleeding complications, and other severe side effects such as coumarin-induced hepatitis. The slow onset and offset of action of VKAs necessitate simultaneous administration of heparins and LMWHs during the induction of anticoagulation as well as during surgical interventions. New anticoagulants are being developed with initial activities in some instances dating back 10 or more years, to overcome these drawbacks of the conventional anticoagulants and, thereby, help to improve patient care.
In most instances, those novel anticoagulants are synthetic small molecules with specific modes of action. They are inhibitors of relevant coagulation enzymes for almost all of which new compounds are being developed. This review focuses on the most advanced group of inhibitors interacting with factor Xa (FXa) or with thrombin. Results from post-operative prevention of thromboembolism and results of dose-finding studies of all indications are not included. Aptamer-based compounds such as TTP889 (Eikelboom et al., 2010) are not included. The included articles were identified in the PubMed database using the names of the individual anticoagulants. Additional online data were used, especially regarding information on the status or cessation of individual clinical trials, mainly from http://www.ClinicalTrials.gov. Data are included as of 1 June 2011.
The new anticoagulants can be grouped by their mode of action that may or may not require antithrombin as cofactor or may involve direct binding and inhibition of coagulation proteases. In this review, pharmacological data and clinical phase III studies for prevention of embolism in patients with atrial fibrillation and for treatment of acute VTE and recurrent thromboembolism are reported in detail. Other studies of earlier clinical phases and in other indications are only shortly reviewed. An overview of the new groups of anticoagulants is shown in Figure 1. Some of the pharmacological effects of the thrombin and FXa inhibitors are compiled in the Table 1.
Figure 1.
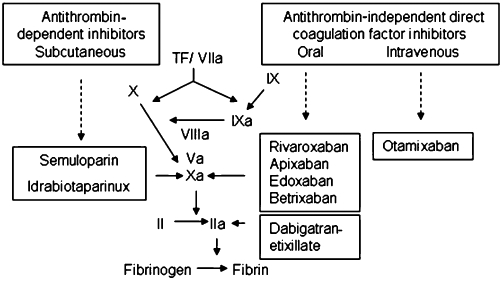
Overview on the specific mode of inhibition of the new anticoagulants.
Table 1.
Pharmacological parameters of the new oral anticoagulants
| Parameter | Dabigatran | AZD 0837 | Rivaroxaban | Apixaban | DU176b | LY517717 | YM 150 | Betrixaban |
|---|---|---|---|---|---|---|---|---|
| Target | FIIa | FIIa | FXa | FXa | FXa | FXa | FXa | FXa |
| MW (Da) | 628 (pro-drug) 472 (active) | na | 436 | 460 | na | 459 | na | 452 |
| Ki (nmol·L−1) | 4.5 | 2–4 | 0.4 | 0.08 | 0.56 | 4.6–6.6 | 0.03 | 0.12 |
| Concentration for doubling of PT (µmol·L−1) | 0.8 | >2 | 0.23 | 3.6 | 0.26 | na | 1.2 | 0.4 |
| Concentration for doubling of aPTT (µmol·L−1) | 0.23 | 0.8 | 0.69 | 7.4 | 0.51 | na | na | na |
| Bioavailability | 6.5% in humans | 22–52% in humans | 57–86% in animals | −66% in humans | 50% in monkeys | 25–82% in animals | 47% | 41–82% in animals |
| tmax (n) | 1.25–3 | 0.7–1.5 | 2–4 | 1–3 | 1–2 | 0.5–4 | na | na |
| Vd (l) | 60–70 | na | −50 | Reported as low | na | na | na | na |
| t1/2(h) | 12–14 | na | 9–13 | 8–15 | 9–11 | 27 | 19 | 5 in dogs |
| Potential drug interactions | Quinidine, amiodarone, potent P-gp inhibitors | Potent CYP3A4 inhibitors | Potent inhibitors of CYP3A4 and P-gp | Potent CYP3A4 inhibitors | na | na | Low potential reported | na |
aPTT = activated partial thromboplastin time; PT = prothrombin time; CYP = cytochrom P450; P-gp = P-glycoprotein; t1/2 = terminal elimination half life; tmax = time to peak plasma concentration; na = not available.
The indirect FXa specific agents, idrabiotaparinux and semuloparin, inhibit FXa following binding to antithrombin, semuloparin, excerting some inhibition of thrombin, too (ratio anti-FXa to antithrombin inhibition, 80 to 1). The oral direct FXa and thrombin inhibitors act through a reversible binding directly and exclusively to the specific coagulation proteases FXa or thrombin.
An advantage of these new anticoagulants is the fixed dosing without the need for anticoagulant monitoring and dose adjustment. This is important for the oral direct FXa and thrombin inhibitors because VKAs have to be dose adjusted according to the target range of the INR between 2 and 3.
All oral small molecule FXa and thrombin inhibitors have been investigated in clinical trials for the posto-perative prevention of VTE. Rivaroxaban and dabigatran have been approved in many countries for the prevention of VTE following total hip and elective knee replacement surgery (Cao et al., 2010; Friedman et al., 2010). Very recently, edoxaban was approved in Japan (http://www.nelm.nhs.uk/en/NeLM-Area/News/2011---April/26/Edoxaban-receives-first-marketing-approval-in-Japan-for-the-prevention-of-VTE-after-major-orthopaedic-surgery-/) and apixaban received a positive statement of the European Medicines Agency (EMA) for this indication (http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion___Initial_authorisation/human/002148/WC500103875.pdf). The other compounds are in different stages of clinical development and are not part of the present review. Idrabiotaparinux and argatroban are not investigated for post-operative prophylaxis of thromboembolism.
Patients with atrial fibrillation suffer from an increased risk of embolic events that can be significantly reduced by VKAs. The limitations for the use of VKAs lead to an estimated underuse in up to 50% of patients requiring an effective anticoagulation (Tulner et al., 2010). The expected ease to use new oral direct coagulation inhibitors may therefore also help to reduce the underuse threat in patients necessitating an effective anticoagulant therapy.
The incidence of embolic events increases with additional cardiovascular risk factors. These are summarized in a scoring system named congestive heart failure, hypertension, age > 75 years, diabetes and stroke (CHADS2) score which has been used in the studies described next. This score consists of a congestive heart failure, hypertension, age > 75 years, diabetes (each 1 point) and stroke (2 points) (Gage et al., 2001). The newer CHA2DS2-VASc score (Lip et al., 2010) was not used in these studies.
Indirect, antithrombin-dependent anticoagulants
Idraparinux and Idrabiotaparinux
The antithrombin-dependent indirect inhibitors in development are idraparinux, idrabiotaparinux and semuloparin. Idraparinux and idrabiotaparinux are polymethylated derivatives of fondaparinux. Their elimination half-life increases from 7 days after single administration up to 60 days after a 6–12 month treatment period (Harenberg, 2009). The development of idraparinux was terminated due to the very long elimination half-life and due to increased bleeding after treatment for longer than 6 months.
Biotinylated Idraparinux, named idrabiotaparinux, is structurally similar to idraparinux, with the addition of a biotin segment. It has the same anticoagulant activity as idraparinux. Avidin exposes a strong affinity to biotin and can be given intravenously to rapidly bind, neutralize and eliminate idrabiotaparinux (Figure 2, Harenberg, 2009).
Figure 2.
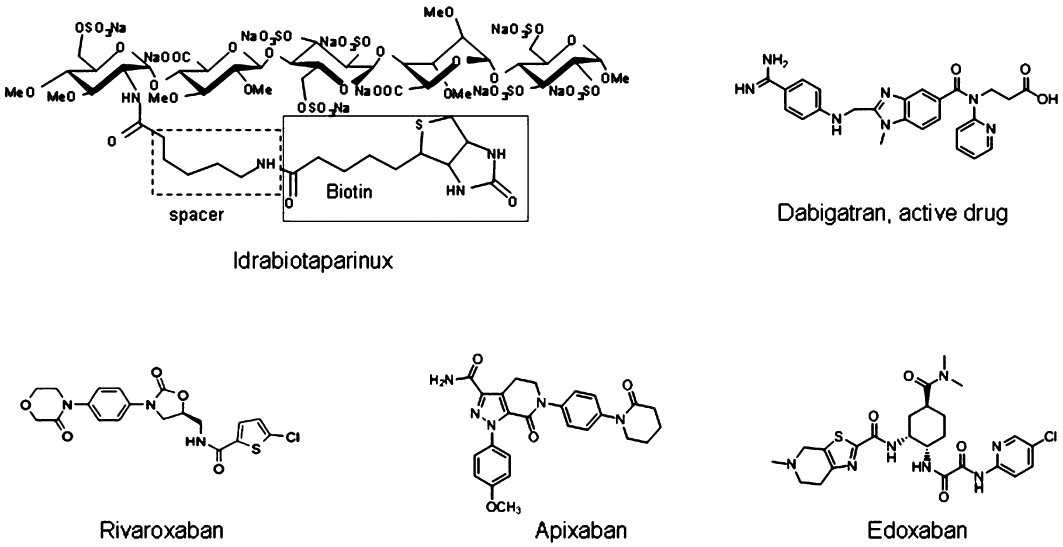
Chemical structures of the anticoagulants with published data of phase III trials for treatment of acute venous thromboembolism and prevention of embolism in atrial fibrillation.
Idrabiotaparinux was investigated in a randomized double-blind trial in 757 patients with symptomatic deep venous thrombosis, comparing equimolar doses of idrabiotaparinux (3 mg) with idraparinux (2.5 mg) subcutaneously once weekly for 6 months. Inhibition of FXa was similar in the two treatment groups. Recurrent VTE occurred in 2.3% of patients randomized to idrabiotaparinux and in 3.2% of patients randomized to idraparinux (not different). The incidence of clinically relevant bleeding was 5.2% in the idrabiotaparinux group and 7.3% in the idraparinux group (not significant). Deaths were not different between the groups (Equinox Investigators, 2011).
The concentration of the two idraparinux and idrabiotaparinux did not differ in the study. They did not reach steady-state conditions after 6 months of therapy. During a 3 month post-study observation period, the decline of the concentration of the anticoagulants was not determined. An increase of the elimination half-life of idraparinux up to 60 days was described during 6 to 12 months' treatment with idraparinux (Harenberg et al., 2008a). This long half-life may be related to the observed bleeding complications (Harenberg et al., 2008b). Such information was not generated during administration of idrabiotaparinux.
Idrabiotaparinux (3.0 mg) once weekly subcutaneously compared with INR-adjusted warfarin for 6–12 months was investigated for the prevention of embolic events in patients with atrial fibrillation. The study was prematurely stopped most probably due to a lack of efficacy/bleeding ratio during therapy with idrabiotaparinux (http://www.en.sanofi.com/binaries/20091221_rdupdate_en_tcm28-26977.pdf).
Semuloparin
Semuloparin, previously named AVE5026, is an ultra-low molecular weight heparin with a mean molecular weight of 2500 Da (range of conventional LMWH 2000–10 000 Da) and an anti-FXa/antithrombin ratio of >30. The following studies using 20 mg semuloparin once daily (OD) subcutaneously have been completed: prophylaxis of post-operative thromboembolism following elective knee or hip surgery or hip fracture (Lassen et al., 2009), extended prophylaxis of VTE in patients having undergone hip fracture surgery (SAVE-HIP3, NCT00709904), prevention of VTE in acutely ill medical patients with restricted mobility (SAVE-VEMED, NCT00714597), prevention of VTE in patients undergoing major abdominal surgery (SAVE-ABDO, NCT00679588), prevention of VTE in cancer patients undergoing chemotherapy (SAVE-ONCO, NCT00694382). Currently, no additional clinical studies are ongoing.
SR123781
SR123781 is a short-acting synthetic hexadecasaccharide for OD injection, which is an indirect antithrombin-dependent inhibitor of Xa coagulation factor (de Kort et al., 2005). The DRIVE phase IIb study evaluating the hexadecasaccharide in the prevention of venous thromboembolic events in patients undergoing total hip replacement demonstrated a correlation between dose and clinical response, with a positive efficacy/safety ratio (Lassen et al., 2008). The SHINE phase IIb study evaluated SR123781 in patients with non-ST elevated acute coronary syndrome. The study demonstrated good safety, with a bleeding rate similar to UFHs with or without abciximab (Harenberg, 2008). New studies are currently not ongoing.
Direct FXa inhibitors
Intravenous direct FXa inhibitor
Otamixaban is a direct potent and selective inhibitor of FXa for intravenous administration. Its half-life is 0.5–1.5 h and it is renally excreted by up to 30%. A phase IIb dose-finding study showed similar TIMI bleeding (major or minor) for all dose groups of otamixaban as compared with intravenous laboratory-adjusted UFH (two to threefold prolongation of the activated partial thromboplastin time) (Cohen et al., 2007). Ongoing studies investigate the influence of renal (NCT01120314) and hepatic impairment (NCT01126086) on the metabolism of otamixaban, and the efficacy compared with UFH plus eptifibatide in patients with unstable angina (NCT01076764).
Oral direct FXa inhibitors
The largest group of anticoagulants consists of direct FXa and direct thrombin inhibitors (DTIs). Direct FXa inhibitors are given intravenously as otamixaban or orally such as rivaroxaban, apixaban, edoxaban, betrixaban. All compounds directly and selectively bind to free FXa and to FXa bound to phospholipids.
Rivaroxaban is a competitive, reversible, direct FXa inhibitor with a Ki of 0.4 nM for purified human FXa and a molecular mass of Mr = 435.89 (Figure 2). After oral administration in man, 60 to 80% are absorbed. Peak plasma levels are achieved in 3 h, and the drug circulates with a half-life of 9 h. Rivaroxaban is excreted by the kidney (66%) and the liver (28%) mainly as unchanged drug. Co-administration of rivaroxaban with food increased the peak plasma concentrations slightly. No additive effects on platelet aggregation were observed during intake of aspirin or the non-steroidal anti-inflammatory drug naproxen, antazid drugs or digoxin. The half-life of rivaroxaban is prolonged in the elderly and in patients with renal impairment (Kubitza and Haas, 2006).
Rivaroxaban was investigated in two independent dose-finding studies for the treatment of acute deep vein thrombosis. It was decided to treat patients with 15 mg twice daily (BID) rivaroxaban for 3 weeks followed by 20 mg OD rivaroxaban over 3–12 months in a large phase III clinical trial. This intervention was compared with body-weight adjusted enoxaparin (1 mg/kg body weight bid) followed by warfarin adjusted to an INR of 2–3 (The EINSTEIN Investigators, 2010). The trial was open, prospective and randomized in patients with acute deep vein thrombosis. During 3–12 months' therapy, 2.1% of patients initially randomized to rivaroxaban developed recurrent VTE (deep vein thrombosis or pulmonary embolism) as compared with 3.0% of patients initially randomized to enoxaparin/warfarin (P < 0.001 for non-inferiority). Major and clinically relevant bleeding complications occurred in 8.1% of patients in both treatment groups.
After termination of prophylaxis of recurrent VTE after initial acute deep vein thrombosis or pulmonary embolism, thromboembolic events re-occur in up to 10 patients within 1 year (Kearon et al., 1999; Schulman et al., 2003). Therefore, the benefit of a prolonged prophylaxis of VTE was investigated in patients following a 6 months' anticoagulation of acute deep vein thrombosis using 20 mg OD rivaroxaban compared with placebo over 12 months for prevention of recurrent VTE in a double-blind study. Patients (7.1%) initially randomized to placebo (n = 594) developed a recurrent VTE during 12 months compared with 1.3% of patients initially randomized to rivaroxaban (n = 602, P = 0.001 for superiority). Severe bleeding complications occurred in 0.7% of patients on rivaroxaban, but in no patient receiving placebo (P = 0.05) (The EINSTEIN Investigators, 2010).
The benefit of an oral anticoagulation with rivaroxaban has been demonstrated for the treatment of acute deep vein thrombosis and recurrent events over a period of 3–12 months as well as the prolonged prophylaxis of recurrent VTE for additional 12 months. The safety of rivaroxaban is comparable with that of warfarin. The benefit on mortality remains to be investigated.
Rivaroxaban is currently investigated for the treatment of acute symptomatic pulmonary embolism and the prevention of recurrent events (NCT00439777).
Patients with atrial fibrillation and a CHADS2 score above 2 were randomly assigned to 20 mg OD rivaroxaban or warfarin adjusted to an INR of 2–3 in a prospective randomized double-blind study (Aalbers, 2011). Seven thousand one hundred thirty-one patients were randomized to rivaroxaban and 7133 patients to warfarin. The mean follow-up was 706 days for rivaroxaban and 708 days for warfarin. The mean CHADS2 score was 3.5 in both treatment groups. Cerebral and non-cerebral embolism occurred in 1.71% per year in patients initially randomized to rivaroxaban and in 2.16% per year in patients initially randomized to warfarin (non-inferiority, P < 0.001, hazard ratio 0.79, 95% confidence interval 0.66–0.96). The incidence of the events of the primary endpoint is shown in Figure 2. The superiority of overall safety in the treatment population (total n = 14 143) was significant (P = 0.015). Based on the intention to treat analysis (n = 14 171), cerebral and non-cerebral embolic events occurred in 2.12% per year in patients initially randomized to rivaroxaban and in 2.42% per year in patients initially randomized to warfarin (P = 0.117). Haemorrhagic strokes occurred at rates of 0.26% per year under rivaroxaban and 0.44% per year under warfarin (hazard ratio 0.59, 95% confidence interval, 0.37–0.93 P = 0.024). Mortality was similar in both treatment groups, with 1.87% per year during treatment with rivaroxaban and 2.21% per year for patients treated with warfarin (P = 0.073). Severe bleeding complications were observed in 3.6% on treatment with rivaroxaban and in 3.45% of patients on treatment with warfarin (P = 0.576). Other bleeding complications were also similar in both treatment groups: 11.8% per 100 years for patients on rivaroxaban and 11.37% per 100 years for patients on warfarin (P = 0.345) (Aalbers, 2011).
The reduction of embolic events by rivaroxaban was less frequently accompanied by bleeding complications compared with warfarin on the basis of the safety analysis. However, using the intention to treat analysis, the incidence of cerebral and non-cerebral embolic events was not different for rivaroxaban compared with warfarin. Intracerebral haemorrhagic bleeding occurred less frequently during treatment with rivaroxaban. This is comparable with the results of the randomized evaluation of long-term anticoagulant therapy (RE-LY) study (see below). Other relevant side effects were not different between the two treatment groups.
Prevention of ischaemic events in patients with unstable angina with rivaroxaban was investigated in a phase IIb study for Mega et al. (2009) and is currently investigated in a phase III trial (Rocket AF Study Investigators, 2010).
Apixaban is a selective and potent (Ki = 0.08 nM) inhibitor of both free and prothrombinase-bound FXa in human plasma (Figure 2). Following oral administration in human, the compound is absorbed to 80% within 3.5 h and is eliminated with a half-life ranging from 8 to 15 h. It is eliminated to about 25% by urinary excretion (Pinto et al., 2007). Apixaban is not a significant inhibitor of CYP enzymes or P-glycoprotein and, therefore, is unlikely to be a significant perpetrator of drug–drug interactions (Wong et al., 2011).
Apixaban is investigated for the treatment of acute deep vein thrombosis or pulmonary embolism (NCT00643201), and for the extended prophylaxis of recurrent events (NCT00633893).
Apixaban was compared with aspirin for the prevention of cerebral and non-cerebral embolism in 5600 patients with atrial fibrillation in whom VKAs were not indicated according to the case history or the decision of the patient or the treating physician [apixaban in patients with atrial fibrillation (AVERROES) study, Connolly et al., 2011]. Apixaban (5 mg BID) (n = 2808) was compared with acetylsalicylic acid (81–324 mg) OD (n = 2791) in a double-blind prospective and randomized trial. A subgroup of patients at an age >80 years, a creatinine clearance <50 mL·min−1 or a risk factor for bleeding, received 2.5 mg BID apixaban. The reasons for unsuitability of VKA therapy were not different between the groups; main reasons were assessment that INR was unlikely to be measured at requested intervals, patients' refusal to take VKAs and multiple reasons for unsuitability of VKA therapy (multiple reasons possible). Patients had a mean CHADS score of 2.0 (apixaban group) or 2.1 (aspirin group). Cerebral and non-cerebral embolism occurred in 1.6% of patients per year during treatment with apixaban and in 3.7% of patients per year under aspirin (hazard ratio 0.45, 95% confidence interval, 0.32–0.62, P < 0.001 for superiority). Death rates were 3.5% per year for patients treated with apixaban and 4.4% per year for patients treated with aspirin (not significant). Major bleeding complications were not different and occurred in 1.4% of patients per year under apixaban and in 1.2% under aspirin (hazard ratio 1.13, 95% confidence interval, 0.74–1.75, P = 0.57). Haemorrhagic stroke, myocardial infarction and death were not different in the treatment groups. Hospitalization for a cardiovascular cause occurred more frequently in patients treated with aspirin (15.9% per year) as compared with apixaban (12.6% per year) (P < 0.001). Minor bleeding complications were higher in patients treated with apixaban (5.0% per year) compared with aspirin (3.6% per year, P = 0.05).
Apixaban was shown to be superior to aspirin for the prevention of cerebral and non-cerebral embolism in patients with atrial fibrillation. The CHADS2 score was low for patients included in this study. The reasons for unsuitability of VKAs are not thoroughly convincing.
In patients with unstable angina, apixaban was investigated in a phase IIb trial for prevention of ischaemic cardiac events (Alexander et al., 2009). A phase II trail was stopped due to major bleeding complications during therapy of apixaban on top of dual antiplatelet therapy (http://www.investor.bms.com/phoenix.zhtml?c=106664&p=irol-newsArticle_print&ID=1498702&highlight=).
Edoxaban (the name originated from Edo as the earlier name of the capital Tokyo of Japan), earlier named as DU-176b, is another orally available, specific and direct inhibitor of FXa (Figure 2). Preclinical studies have demonstrated bioavailability of approximately 60% in monkeys based on 14C-edoxaban detected in urine (Zafar et al., 2007).
Edoxaban (NCT00986154) is investigated for the treatment of acute deep vein thrombosis and pulmonary embolism.
Prophylaxis of embolism in patients with atrial fibrillation is currently investigated using edoxaban (NCT not available at present) and betrixaban (NCT00742859).
YM-150, LY517717 and TAK-421 are other oral synthetic, low molecular weight, direct FXa inhibitor, with comparable pharmacokinetic properties as the other oral FXa inhibitors (Eriksson et al., 2009; Kawamura et al., 2010). They have been investigated in phase IIb trials in different indications (Agnelli et al., 2007; Eriksson et al., 2007; Turpie et al., 2009; Weitz et al., 2010). At present, no phase III clinical trials are ongoing with these anticoagulants.
Direct thrombin inhibitors
The most relevant synthetic, small molecule DTIs are argatroban, ximelagatran and dabigatran etexilate (Figure 2), and AZD 0837. They bind specifically to the active centre of thrombin and inactivate free and fibrin-bound thrombin (Hauptmann and Stürzebecher, 1999). After inactivation of thrombin, the inhibitors reverse from their binding site, thereby leaving a small amount of free and active thrombin available to control haemostasis (Gustafsson and Elg, 2003). The vascular binding differs from that of the irreversible, non-covalent binding of the DTIs hirudin and hirologs (Direct Thrombin Inhibitor Trialists' Collaborative Group, 2002). AZD 0837 is a prodrug of the direct inhibitor AR-H067637, with a concentration that dependently inhibits tissue factor-induced generation of free thrombin. The inhibition constant is in the same concentration range as reported for melagatran and dabigatran (Deinum et al., 2009).
Oral direct thrombin inhibitors
Dabigatran, an oral direct thrombin inhibitor, has been investigated for the treatment of acute deep vein thrombosis and pulmonary embolism and the prevention of recurrent events over 6 months compared with warfarin in a double-blind prospective randomized trial (Schulman et al., 2009). Patients were initially treated with 150 mg BID dabigatran or body weight adjusted enoxaparin (1 mg·kg−1 body weight BID) or with warfarin adjusted to an INR of 2–3. 2.4% of patients initially randomized to dabigatran (n = 1274) and 2.1% of patients initially randomized to warfarin (n = 1265) developed recurrent thromboembolic events. The study hypothesis of non-inferiority was fulfilled (P = 0.001, relative risk 1.1, 95% confidence interval 0.65–1.86).
Severe bleeding complications recurred in 1.6% of patients treated with dabigatran and in 1.9% of patients treated with warfarin. Minor bleeding complications occurred in 16.1% of patients treated with dabigatran and in 21.9% of patients treated with warfarin (P = 0.01). Dyspepsia was observed in 3.6% of patients treated with dabigatran and in 0.7% of patients treated with warfarin (P = 0.001). Other side effects were not different between groups. Liver enzymes over time were not different between the treatment groups. Acute coronary events did not differ between both treatment groups (0.4% vs. 0.2%).
Dabigatran has been proven to be effective and safe for the treatment of acute VTE and prevention of recurrent events. The advantage of this study over the analogous study on rivaroxaban is its double-blind study design. A minor limitation in the study is the occurrence of dyspepsia.
Studies on an extended prophylaxis of recurrent events for additional 18 months are waiting for completion (NCT00558259: dabigatran vs. warfarin; NCT00558259: dabigatran vs. placebo).
AZD 0837 is not investigated for this indication.
In a phase III trial, RE-LY compares the efficacy and safety of two blinded doses of dabigatran etexilate with open-label warfarin for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (Connolly et al., 2009). It was a prospective, multicentre, randomized, open-label, controlled parallel group, non-inferiority trial. Systemic embolic events occurred in 1.69% per year in patients randomized to warfarin, 1.53% per year in patients randomized to 110 mg dabigatran BID and 1.11% per year in patients randomized to 150 mg dabigatran BID [relative risk (RR) 0.91, CI 0.74 to 1.11, P < 0.001 for non-inferiority with 110 mg dabigatran BID; and RR 0.66, CI 0.53 to 0.82, P < 0.001 for superiority with 150 mg dabigatran BID]. Major bleeding occurred in 3.36% of patients per year on warfarin, in 2.71% of patients per year on 110 mg dabigatran BID (P = 0.003),and 3.11% of patients per year on 150 mg dabigatran BID [not significant (NS)]. The mortality rates were 4.13, 3.75 and 3.64% per year in the warfarin, 110 and 150 mg dabigatran groups (NS and P = 0.051 vs. warfarin), respectively. Dyspepsia occurred in 348 patients (5.8%) in the warfarin group and in 707 patients (11.8%) and 688 patients (11.3%) in the 110 and 150 mg dabigatran groups, respectively (P < 0.001 each). The rates of myocardial infarction were 0.53% per year with warfarin, 0.72% per year with 110 mg BID dabigatran (RR 1.35, 95% CI 0.98 to 1.87; P = 0.07) and 0.74% per year with 150 mg BID dabigatran (RR 1.38, 95% CI 1.00 to 1.91; P = 0.048).
The dabigatran treatment in patients with atrial fibrillation who completed RE-LY trial (RE-LY-ABLE) is an extension trial of dabigatran treatment in patients who successfully completed the RE-LY study. The trial will provide an opportunity for about 8000 patients to remain on blinded 110 mg BID and 150 mg BID dabigatran for 12–36 months without switching to VKA. The data will provide additional safety information on patients with long-term exposure to dabigatran (NCT00808067).
The RE-LY study compares two doses of dabigatran with warfarin. For the first time, an anticoagulant regimen of 150 mg BID dabigatran was proven to be more effective in reducing embolic events in patients with atrial fibrillation compared with warfarin. In addition, cerebral haemorrhage occurred significantly less with both doses of dabigatran compared with INR-adjusted warfarin. The higher incidence of myocardial infarction was outweighed by the benefits of dabigatran on the other endpoints. The continuation of patients in the RE-LY-ABLE study will generate additional safety data for dabigatran. The Food and Drug Administration has approved the 150 mg BID dabigatran dose for the prevention of embolism in patients with atrial fibrillation as well as a dose of 75 mg BID dabigatran for patients with impaired renal function (creatinine clearance 20–50 mL·min−1).
Currently, the costs of the drug dabigatran for the prevention of stroke in patients with atrial fibrillation currently exceed that of warfarin by about 7000-fold. Therefore, the quality-adjusted survival, costs and cost-effectiveness of dabigatran were compared with those for warfarin adjusted to an INR of 2–3 using the data of the patients included into the RE-LY study (Freeman et al., 2011). The Markov decision model was used for this analysis. The estimated costs were made on the basis of pricing in the UK. Warfarin targeted at an INR of 2–3, dabigatran 110 mg BID and dabigatran 150 mg BID was compared. Outcome measures were quality-adjusted life years, costs in US$ (year 2008) and incremental cost-effectiveness ratios. The model was sensitive to the costs of dabigatran but was relatively insensitive to other model inputs. According to this model, high-dose dabigatran was more cost-effective than low-dose dabigatran. The analysis showed that treatment with dabigatran could be cost-effective compared with warfarin for the prevention of cerebral and non-cerebral embolism in patients above 65 years and with an increased risk of stroke. The main limitation of this investigation was the assumption of no change of the costs for dabigatran.
The prevention of ischaemic events in patients with acute coronary syndromes was investigated in a phase IIb study using dabigatran in addition to aspirin and clopidogrel. Dabigatran, in addition to dual antiplatelet therapy, was associated with a dose-dependent increase in bleeding events and significantly reduced coagulation activity in patients with a recent myocardial infarction (Oldgren et al., 2011).
Comments
The cost-effectiveness analysis provides substantial information for the decision which dose of dabigatran or if warfarin should be used in patients with atrial fibrillation based on the individual risk factors for haemorrhage and thromboembolism. However, the analysis has some limitations:
The price of dabigatran, presently marketed in Europe for short-term prophylaxis of post-operative prophylaxis of VTE, will drop if the indication for prophylaxis of embolic events in patients with atrial fibrillation will be approved by the EMA.
The price for dabigatran will drop again after the approval of another direct coagulation inhibitor (rivaroxaban or apixaban or both or more compounds) for this indication.
The results of the AVERROES study indicate that apixaban will reduce the treatment costs compared with acetylsalicylic acid or compared with no therapy (current underuse).
Perspectives
At present, several studies have been published recently for the treatment of deep vein thrombosis and the prevention of embolic events in patients with atrial fibrillation. Both are the main new indications for new oral anticoagulants to overcome the drawbacks of conventional anticoagulation.
The major drawbacks of the new oral anticoagulants will become visible only after market approval. They comprise high daily treatment costs, fear of unforeseen and unknown side effects, and – in patients comfortably performing therapy with VKAs – fear of switching to an oral anticoagulant with less experience.
The current underuse of VKAs will be reduced by the availability of new oral anticoagulants. Younger patients will be more prone to be treated with the new anticoagulants due to the simplicity of the treatment. The availability of several new oral anticoagulants should help to competitively reduce the daily price for the drugs. Additional information including meta-analyses will improve the knowledge about the added values of the new oral anticoagulants compared with conventional ones.
Authors' opinion
The new oral anticoagulants present a new and promising therapeutic option for patients requiring oral anticoagulation. Patients with currently ineffective anticoagulation will specifically benefit from the new classes of anticoagulants. Special attention has to be made to an adequate pricing and for specific indications to determine the anticoagulant effect.
Acknowledgments
The authors did not receive financial support for the preparation of the manuscript.
Glossary
- AVERROES
Apixaban in patients with atrial fibrillation
- BID
twice daily
- CHADS
congestive heart failure, hypertension, age > 75 years, diabetes and stroke
- CHADS2-VASc
congestive heart failure, hypertension, age > 75 years, diabetes and stroke (2 points), vascular disease
- DRIVE
dose ranging study in elective total hip replacement surgery
- DTI
direct thrombin inhibitor
- EINSTEIN
oral direct factor Xa inhibitor rivaroxaban in patients with acute symptomatic deep-vein thrombosis
- EMA
European Medicines Agency
- FXa
factor Xa
- ICER
incremental cost effectiveness ratios
- INR
international normalized ratio
- LMWH
low molecular weight heparin
- NS
not significant
- OD
once daily
- QALYs
quality adjusted life years
- RE-LY
randomized evaluation of long term anticoagulant therapy with dabigatran etexilate
- RE-LY-ABLE
dabigatran treatment in patients with atrial fibrillation who completed RE-LY trial
- SAVE-ABDO
semuloparin AVE5026 abdominal surgery
- SAVE-HIP
semuloparin AVE5026 elective hip repalacement surgery
- SAVE-ONCO
semuloparin AVE5026 oncology
- SAVE-VEMED
semuloparin AVE5026 venous thromboembolism in medical patients study
- SHINE
SR123781 in patients with unstable angina
- UFH
unfractionated heparin
- VKA
vitamin K antagonist
- VTE
venous thromboembolism
Conflict of interest
The authors do not have to declare any conflict of interest relating to the manuscript.
References
- Aalbers J. Special Assignment Editor Rivaroxaban equals warfarin treatment in atrial fibrillation patients at high risk of stroke. Cardiovasc J Afr. 2011;21:342–343. [PMC free article] [PubMed] [Google Scholar]
- Agnelli G, Haas S, Ginsberg JS, Krueger KA, Dmitrienko A, Brandt JT. A phase II study of the safety and efficacy of a novel oral fXa inhibitor (LY517717) for the prevention of venous thromboembolism following TKR or THR. J Thromb Haemost. 2007;5:746–753. doi: 10.1111/j.1538-7836.2007.02436.x. [DOI] [PubMed] [Google Scholar]
- Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, Dellborg M, et al. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 2009;119:2877–2885. doi: 10.1161/CIRCULATIONAHA.108.832139. [DOI] [PubMed] [Google Scholar]
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15:244–252. doi: 10.18553/jmcp.2009.15.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RC, Thomas W, Meade PB, Berger M, Ezekowitz CM, O'Connor DA, et al. The primary and secondary prevention of coronary artery disease: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:776S–814S. doi: 10.1378/chest.08-0685. [DOI] [PubMed] [Google Scholar]
- Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2010;66:1099–1108. doi: 10.1007/s00228-010-0889-z. [DOI] [PubMed] [Google Scholar]
- Cohen M, Bhatt DL, Alexander JH, Montalescot G, Bode C, Henry T, et al. on behalf of the SEPIA-PCI Trial Investigators. Randomized, double-blind, dose-ranging study of otamixaban, a novel, parenteral, short-acting direct factor Xa inhibitor, in percutaneous coronary intervention: the SEPIA-PCI trial. Circulation. 2007;115:2642–2651. doi: 10.1161/CIRCULATIONAHA.106.653428. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. The AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- Deinum J, Mattsson C, Inghardt T, Elg M. Biochemical and pharmacological effects of the direct thrombin inhibitor AR-H067637. Thromb Haemost. 2009;101:1051–1059. [PubMed] [Google Scholar]
- Direct Thrombin Inhibitor Trialists' Collaborative Group. Direct thrombin inhibitors in acute coronary syndromes: principal results of a meta-analysis based on individual patients. Lancet. 2002;359:294–302. doi: 10.1016/S0140-6736(02)07495-0. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Zelenkofske SL, Rusconi CP. Coagulation factor IXa as a target for treatment and prophylaxis of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2010;30:382–387. doi: 10.1161/ATVBAHA.110.203117. [DOI] [PubMed] [Google Scholar]
- Equinox Investigators. Efficacy and safety of once weekly subcutaneous idrabiotaparinux in the treatment of patients with symptomatic deep venous thrombosis. J Thromb Haemost. 2011;9:92–99. doi: 10.1111/j.1538-7836.2010.04100.x. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Turpie AG, Lassen MR, et al. for the ONYX STUDY GROUP A dose escalation study of YM150, an oral direct factor Xa inhibitor, in the prevention of venous thromboembolism in elective primary hip replacement surgery. J Thromb Haemost. 2007;5:1660–1665. doi: 10.1111/j.1538-7836.2007.02644.x. [DOI] [PubMed] [Google Scholar]
- Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [DOI] [PubMed] [Google Scholar]
- Friedman RJ, Dahl OE, Rosencher N, Caprini JA, Kurth AA, Francis CW, et al. RE-MOBILIZE, RE-MODEL, RE-NOVATE Steering Committees. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res. 2010;126:175–182. doi: 10.1016/j.thromres.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- Gustafsson D, Elg M. The pharmacodynamics and pharmacokinetics of the oral direct thrombin inhibitor ximelagatran and its active metabolite melagatran. Thromb Res. 2003;109:S9–S15. doi: 10.1016/s0049-3848(03)00249-4. [DOI] [PubMed] [Google Scholar]
- Harenberg J. Development of new anticoagulants: present and future. Semin Thromb Hemost. 2008;34:779–793. doi: 10.1055/s-0029-1145260. [DOI] [PubMed] [Google Scholar]
- Harenberg J. Development of idraparinux and idrabiotaparinux for anticoagulant therapy. Thromb Haemost. 2009;102:811–815. doi: 10.1160/TH09-08-0555. [DOI] [PubMed] [Google Scholar]
- Harenberg J, Jörg I, Vukojevic Y, Mikus G, Weiss C. Anticoagulant effects of idraparinux after termination of therapy for prevention of recurrent venous thromboembolism: observations from the van Gogh trials. Eur J Clin Pharmacol. 2008a;64:555–563. doi: 10.1007/s00228-008-0463-0. [DOI] [PubMed] [Google Scholar]
- Harenberg J, Vukojevic Y, Mikus G, Joerg I, Weiss C. Long elimination half-life of idraparinux may explain major bleeding and recurrent events of patients from the van Gogh trials. J Thromb Haemost. 2008b;6:890–892. doi: 10.1111/j.1538-7836.2008.02943.x. [DOI] [PubMed] [Google Scholar]
- Hauptmann J, Stürzebecher J. Synthetic inhibitors of thrombin and factor Xa: from bench to bedside. Thromb Res. 1999;93:203–241. doi: 10.1016/s0049-3848(98)00192-3. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:141S–159S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Konishi N, Hiroe K, Shofuda K, Imaeda Y, Fujimoto T, et al. Antithrombotic and anticoagulant profiles of TAK-442, a novel factor Xa inhibitor, in a rabbit model of venous thrombosis. J Cardiovasc Pharmacol. 2010;56:156–161. doi: 10.1097/FJC.0b013e3181e2bfcf. [DOI] [PubMed] [Google Scholar]
- Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–917. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- de Kort M, Buijsman RC, van Boeckel CA. Synthetic heparin derivatives as new anticoagulant drugs. Drug Discov Today. 2005;10:769–779. doi: 10.1016/S1359-6446(05)03457-4. [DOI] [PubMed] [Google Scholar]
- Kubitza D, Haas S. Novel factor Xa inhibitors for prevention and treatment of thromboembolic diseases. Expert Opin Investig Drugs. 2006;15:843–855. doi: 10.1517/13543784.15.8.843. [DOI] [PubMed] [Google Scholar]
- Lassen MR, Dahl O, Mismetti P, Zielske D, Turpie AG. SR123781A: a new once-daily synthetic oligosaccharide anticoagulant for thromboprophylaxis after total hip replacement surgery: the DRIVE (Dose Ranging Study in Elective Total Hip Replacement Surgery) study. J Am Coll Cardiol. 2008;51:1498–1504. doi: 10.1016/j.jacc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Lassen MR, Dahl OE, Mismetti P, Destrée D, Turpie AG. AVE5026, a new hemisynthetic ultra-low-molecular-weight heparin for the prevention of venous thromboembolism in patients after total knee replacement surgery–TREK: a dose-ranging study. J Thromb Haemost. 2009;7:566–572. doi: 10.1111/j.1538-7836.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29–38. doi: 10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, et al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr113. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Orwat MJ, Koch S, Rossi KA, Alexander RS, Smallwood A, et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro- 1H-pyrazolo[3,4-c]pyridine-3-carboxamide (Apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem. 2007;50:5339–5356. doi: 10.1021/jm070245n. [DOI] [PubMed] [Google Scholar]
- ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–347. doi: 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Schulman S, Wåhlander K, Lundström T, Clason SB, Eriksson H THRIVE III Investigators. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med. 2003;349:1713–1721. doi: 10.1056/NEJMoa030104. [DOI] [PubMed] [Google Scholar]
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- Tulner LR, Van Campen JP, Kuper IM, Gijsen GJ, Koks CH, Mac Gillavry MR, et al. Reasons for undertreatment with oral anticoagulants in frail geriatric outpatients with atrial fibrillation: a prospective, descriptive study. Drugs Aging. 2010;27:39–50. doi: 10.2165/11319540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Turpie AG, Bauer KA, Davidson BL, Fisher WD, Gent M, Huo MH, et al. EXPERT Study Group. A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT) Thromb Haemost. 2009;101:68–76. [PubMed] [Google Scholar]
- Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:340S–380S. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- Weitz JI, Cao C, Eriksson BI, Fisher W, Kupfer S, Raskob G, et al. A dose-finding study with TAK-442, an oral factor Xa inhibitor, in patients undergoing elective total knee replacement surgery. Thromb Haemost. 2010;104:1150–1157. doi: 10.1160/TH10-05-0273. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis. 2011;31:478–492. doi: 10.1007/s11239-011-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar MU, Vorchheimer DA, Gaztanaga J, Velez M, Yadegar D, Moreno PR, et al. Antithrombotic effects of factor Xa inhibition with DU-176b: phase-I study of an oral, direct factor Xa inhibitor using an ex-vivo flow chamber. Thromb Haemost. 2007;98:883–888. doi: 10.1160/th07-04-0312. [DOI] [PubMed] [Google Scholar]


