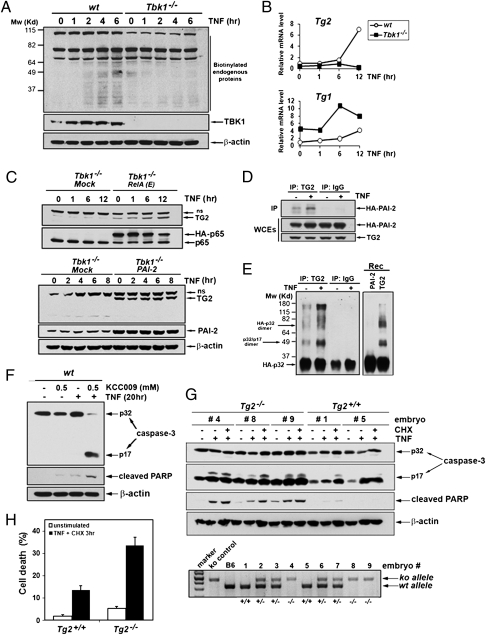
Fig. 4.
TG2 is a TBK1-dependent antiapoptotic factor. (A) In vivo transamidation activity was determined in WT and Tbk1−/− MEFs metabolically labeled with BP and stimulated with TNF for the indicated times. Cell extracts were prepared, and biotin-conjugated proteins were detected by immunoblotting using anti–streptavidin-HRP (Pierce). (B) Induction of Tg gene expression was determined by qRT-PCR analysis of mRNAs from WT and Tbk1−/− MEFs stimulated with TNF for the indicated times. (C) Endogenous TG2 protein amounts were determined by immunoblotting in extracts from Tbk1−/− MEFs expressing RelA(E) (Upper), PAI-2 (Lower), or an “empty” retrovirus (mock) and treated with TNF for the indicated times (ns, nonspecific). (D) Coimmunoprecipitation of PAI-2 with TG2 was examined in MEFs stably expressing HA-PAI-2 and human TG2 that were left untreated or were stimulated with TNF for 8 h. Control immunoprecipitations were performed with nonimmune IgGs. (E) In vitro transamidation assay was performed with TG2 or control immunoprecipitates prepared from untreated or TNF-stimulated Tbk1−/− MEFs expressing TG2 and untagged PAI-2 or with recombinant proteins (rec) using HA-procaspase-3 as a substrate. (F) Wt MEFs were treated with TNF (20 h) with or without the transglutaminase-specific inhibitor KCC009 (0.5 mM). Caspase-3 activation, PARP cleavage, and protein expression were determined by immunoblotting. (G) WT and Tg2−/− primary MEFs prepared from littermate embryos were left untreated or treated with TNF alone (20 h) or TNF plus CHX (6 h). Caspase-3 activation and apoptosis were determined as detailed earlier. Genotyping (Lower) was performed by PCR amplification as described (31). (H) The extent of cell death in WT and Tg2−/− MEFs that were stimulated for 3 h with TNF plus CHX was quantified by staining with PI.