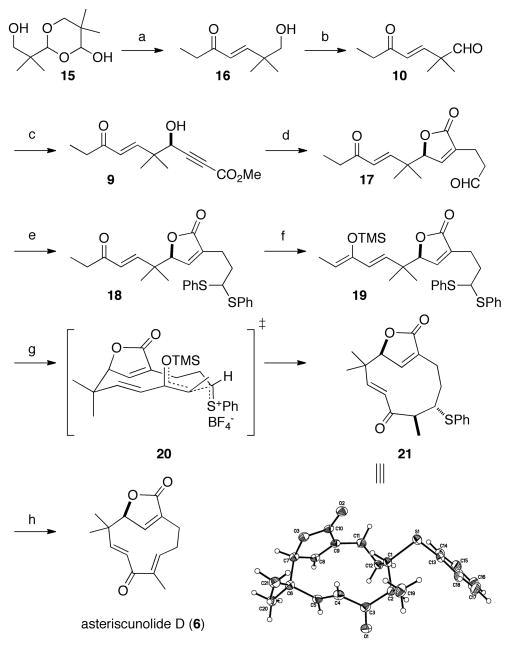
Scheme 1.
Synthesis of asteriscunolide D (6)
a (a) (MeO)2P(O)CH2C(O)CH2CH3 (1.0 equiv), LiCl (1.8 equiv), i-Pr2NEt (1.8 equiv), CH3CN, 4 h, 91%. (b) (COCl)2 (1.5 equiv), DMSO (3.0 equiv), Et3N (6.0 equiv), CH2Cl2, −78 °C, 99% (c) (S,S)-11 (20 mol %), Me2Zn (2.95 equiv), methyl propiolate (2.8 equiv), toluene, 4 °C, 36 h, 83% yield, 84% ee. (d) allyl alcohol (1.5 equiv), CpRu(CH3CN)3PF6 (5 mol %), CSA (0.25 equiv), THF, acetone, 50 °C, 4.5 h, 55%. (e) thiophenol (2.0 equiv), BF3·Et2O (15 mol %), CH2Cl2, 15 h, 79%. (f) TMSOTf (1.4 equiv), i-Pr2NEt (1.5 equiv), CH2Cl2, 0 °C, 1.5 h, 90%, (g) DMTSF (1.2 equiv), 4 Å MS, CH2Cl2, −30 °C, 1.5 h, 32–41%. (h) Me3OBF4 (1.8 equiv), CH2Cl2, 16 h; then i-Pr2NEt (1.8 equiv), 40 °C, 6 h, 82%.