Table 2.
Reaction scope with nitroalkene derivativesa
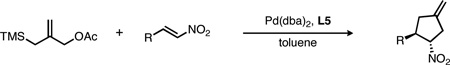 | |||||
|---|---|---|---|---|---|
| entry | R = | product |
T, °C |
% yield |
% ee |
| 1 | 3-bromophenyl | 7 | 50 | 63 | 94 |
| 2 | 4-chlorophenyl | 8 | 50 | 65 | 95 |
| 3 | 2-methylphenyl | 9 | 23 | 91 | 93 |
| 4 | 4-methylphenyl | 10 | 50 | 82 | 89 |
| 5 | 4-methoxyphenyl | 11 | 23 | 72 | 94 |
| 6 | 1-naphthyl | 12 | 23 | 67 | 92 |
| 7 | 2-naphthyl | 13 | 50 | 82 | 93 |
| 8 | 3,4-methylenedioxyphenyl | 14 | 50 | 80 | 91 |
| 9 | 2-furyl | 15 | 23 | 66 | 88 |
| 10 | 3-furyl | 16 | 50 | 58 | 87 |
| 11 | 2-thiophenyl | 17 | 23 | 75 | 91 |
| 12 | N-Boc-3-indolyl | 18 | 50 | 91 | 86 |
| 13 | n-propyl | 19 | 50 | 88 | 83 |
| 14 | cyclohexyl | 20 | 50 | 97 | 93 |
| 15 | t-butyl | 21 | 50 | 97 | 88 |
| 16 | (E)-styrenyl | 22 | 50 | 53 | 93 |
All reactions were conducted at 0.15 M in toluene at 50 °C, with 1.6 equiv of 1a, 5% Pd(dba)2 and 10% L5. Yields are isolated values; ee’s were determined by chiral HPLC.
