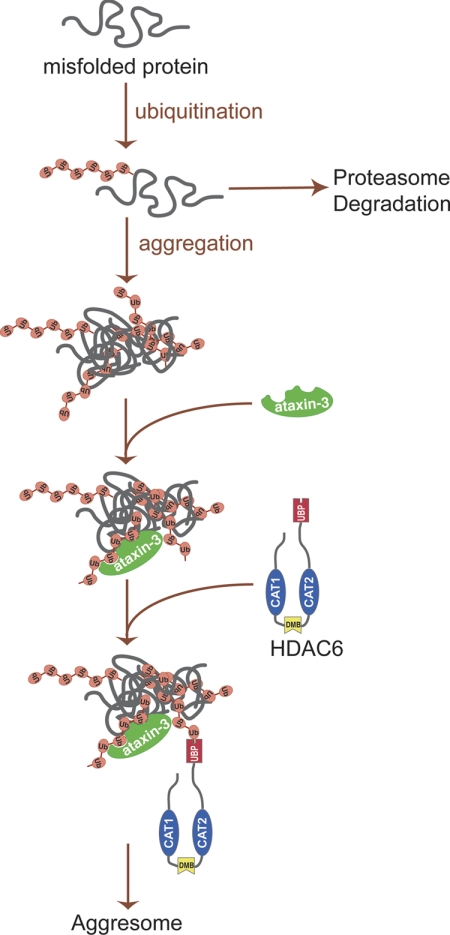
FIGURE 5.
A schematic representation of aggresome formation. Under normal conditions, polyubiquitinated misfolded proteins are efficiently degraded by ubiquitin proteasome. When ubiquitin proteasome is disrupted or overwhelmed, polyubiquitinated misfolded proteins form aggregates. Deubiquitinase ataxin-3 interacts with polyubiquitinated protein aggregates, generating unanchored ubiquitin or ubiquitin chains. HDAC6 recognizes and binds these unanchored ubiquitin C-terminal tails in protein aggregates and recruits them to dynein motor complexes that subsequently transport the aggregated cargo to the aggresomes.