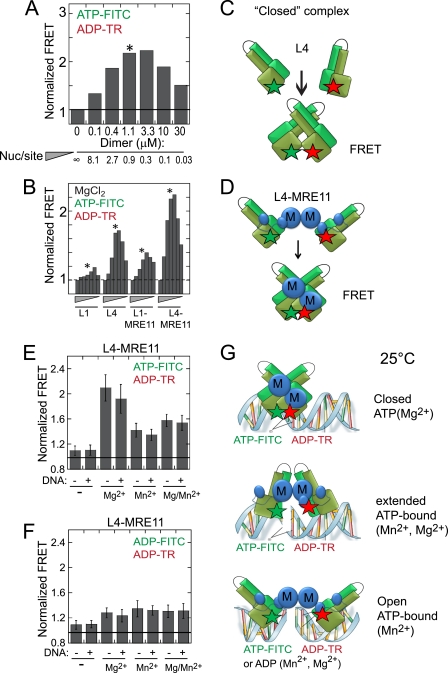
FIGURE 2.
ATP binding closes both RAD50 and RAD50-MRE11 complexes. A and B, the normalized FRET signal for ATP-bound L1, L4, or their complexes with MRE11 is shown. A, an example of the normalized FRET analysis is shown. In the assays each BODIPY-labeled nucleotide was held constant at 1 μm, and purified protein (expressed as dimers) was added in increasing concentrations. As protein concentration increases, the nucleotide per site ratio decreases as indicated by the inverted triangles. The star indicates that the maximum FRET intensity occurred at a nucleotide per binding site ratio of ∼1:1. B, shown is the FRET analysis in A for ATP-FITC and ATP-TR binding to L1, L4, L1-MRE11, or L4-MRE11 protein in the presence of Mg2+. Reactions containing purified protein, nucleotides, and 5 mm MgCl2 were incubated in a 20-μl reaction volume for 5 min at 25 °C before measurement. C and D, shown is a schematic diagram of the ATP-driven closed complexes derived from the FRET results for L4 (C) and L4-MRE11 (D). The green and red stars are the ATP-FITC- and ATP-TR-labeled nucleotides, respectively. M (blue) is MRE11; light and dark green dimer is RAD50. Close stars indicate FRET, whereas the separated stars represent no FRET. The fluorescence intensity of a donor (excitation 470 and emission 522 nm) and intensity of energy transfer (excitation 470 and emission 615 nm) were recorded in a TECAN plate reader. E, shown are FRET results of B in which 5 mm ATP (Mg2+), ATP (Mn2+), a stoichiometric ratio of both, or no ion (−) were added to the L4 or L4-MRE11 in the presence (+) or absence (−) of DNA (40-bp double-stranded oligonucleotide). F, details are the same as E except that ADP-FITC and ADP-TR were the added nucleotides. G, shown is a schematic diagram for the RAD50-MRE11 complexes derived from the FRET results depicting the conformation of ATP-bound complexes. Top, the L4-MRE11 complex is closed when RAD50 is bound with ATP (Mg2+); bottom, complex is open when L4-MRE11 is bound with ATP (Mn2+) or ADP (in the presence of any added divalent ion); middle, an intermediate conformation or mixture when L4-MRE11 is bound equally with ATP (Mg2+) and ATP (Mg2+). Red and green stars represent ATP-TR- and ATP-FITC-labeled nucleotide, respectively.