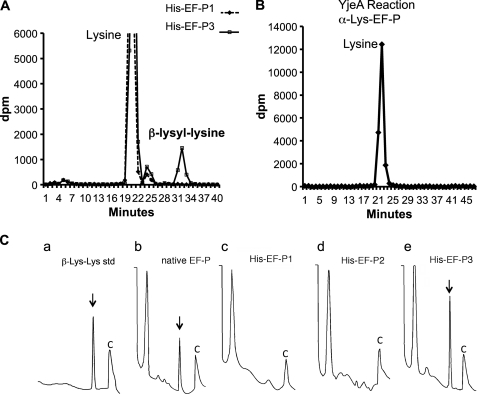
FIGURE 4.
Ion exchange chromatographic identification of β-lysyl-lysine in the Pronase digest of native EF-P and recombinant His-EF-P from cells coexpressing His-EF-P, YjeA, and YjeK. A, [3H]lysine-labeled His-EF-P proteins were purified from E. coli and were exhaustively digested with Pronase. The digests were analyzed by ion exchange chromatography as described under “Experimental Procedures.” A radioactive peak corresponding to a reference β-lysyl-lysine standard was found in the digest of in vivo modified His-EF-P3 but not in the digest of unmodified His-EF-P1. B, no radioactive β-lysyl-lysine was found in the digest of α-lysyl-EF-P formed in an in vitro YjeA reaction. Only [3H]lysine was found, indicating that the α-lysyl-lysine bond is cleaved by Pronase. C, standard β-lysyl-lysine (a) and exhaustive Pronase digests of native (b) or recombinant EF-P proteins (c–e) were analyzed by ion exchange chromatography, and amino acids or peptides were measured by a fluorometric detection method (32), as described under “Experimental Procedures.” Only the latter part of the chromatogram (30–70 min) is shown with two reference peaks before and after the β-lysyl-lysine (indicated by an arrow or arrowhead). C, a reference peak (buffer change) at the end of each run.