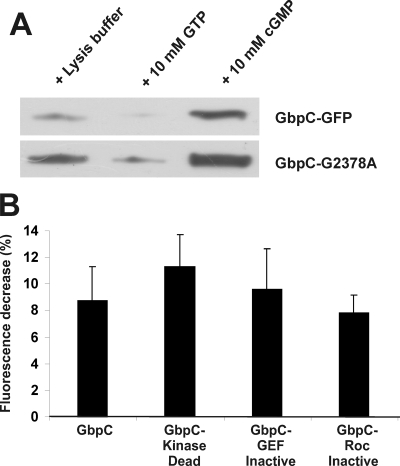
FIGURE 5.
Protein localization and intramolecular signaling of GbpC are independent processes. A, GbpC-GFP and GbpC-G2378A-GFP were expressed in gbpC-null cells. Lysates were prepared, and proteins were pulled down with GTP-agarose beads in the absence or presence of cGMP or GTP, respectively. Bound proteins were visualized on a Western blot analysis using anti-GFP antibodies. cGMP-stimulated GTP binding was still present in the G2378A mutant at comparable levels with the wild-type protein. Representative data for at least three independent experiments on different days are presented. B, quantification of the maximal cytoplasmatic fluorescence decrease after cAMP stimulation for GbpC and the previously described GbpC-KinaseDead, GbpC-GefInactive, and GbpC-RocInactive mutants expressed in gbpC-null cells. Cells were treated as described in Fig. 1.