Figure 2.
Raf-1 Affects VEC-Mediated AJ Formation
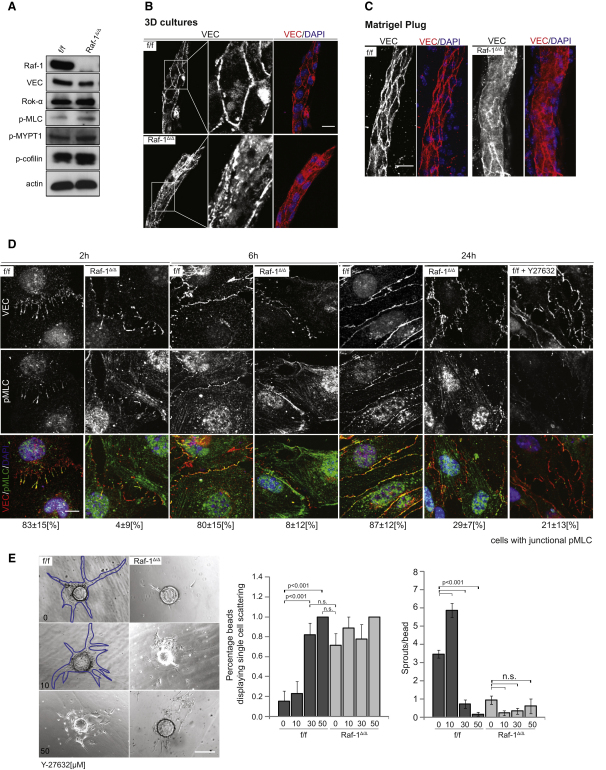
(A) Rok-α substrates are slightly hyperphosphorylated in continuously growing Raf-1Δ/Δ pMECs. Expression of Raf-1, VEC, and Rok-α, and phosphorylation of Rok-α downstream targets were detected by immunoblotting.
(B and C) VEC fails to localize at cell-cell borders in sprouts formed by Raf-1Δ/Δ pMECs in 3D cultures (B) and in matrigel plugs (C). VEC patterns (red) and nuclei (DAPI, blue) were analyzed by confocal microscopy.
(D) Delayed formation of VEC-containing AJ and mislocalization of activated myosin (pMLC) in Raf-1Δ/Δ iMECs. iMECs were seeded on chamber slides in the presence of FGF-2 (50 ng/ml) and fixed at the indicated time points. The localization of VEC (red) and pMLC (green) was analyzed by confocal microscopy. Nuclei were stained with DAPI (blue). Note the consistent colocalization of VEC and pMLC in the f/f iMECs and the lack of colocalization in the Raf-1Δ/Δ cells or in cells treated with the Rok inhibitor (20 μM Y-27632). The numbers below the pictures indicate the percentage of cells positive for junctional pMLC, ± SD.
(E) Rok inhibition does not rescue the sprouting defect of Raf-1Δ/Δ pMECs. Y-27632 was added to the fibrin gels at indicated concentrations. Sprout formation was analyzed as in Figure 1A. Note the increased amount of individual cells scattered around the microcarrier in the f/f cultures treated with 50 μM Y-27632. A quantification of the results is shown in the right panel (minimum n = 25 microcarriers per genotype and treatment; error bars, SE of the mean). Blue traces have been added to two of the images lacking contrast to highlight the profile of the sprouts. Scale bars (B) and (C), 20 μm; (D), 10 μm; and (E), 200 μm. p < 0.001, according to Student's t tests. n.s., not significant.