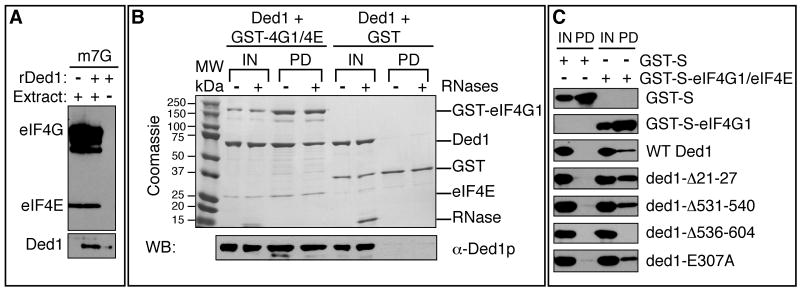
Figure 4. The C terminal assembly domain of Ded1 is critical for interaction with eIF4E/eIF4G.
A. Yeast translation extract and rDed1 were incubated with m7G-sepharose and bound proteins identified by immunoblotting with antibodies against Ded1, eIF4E, or eIF4G. B. Interactions between recombinant Ded1 and GST or co-purified GST-eIF4G/eIF4E were tested by immunoprecipitation of eIF4G by glutathione in the presence of RNases. Proteins were detected by coomassie staining (top) and western blot (for Ded1, bottom). The input lanes show 5% of the reaction volume; pull-down lanes show the entire sample. C. As in Figure 4B, except that bound proteins were analyzed by western blot with anti-S antibody (for GST and GST-eIF4G1) or anti-Ded1 antibody. See also Figure S5, S6.