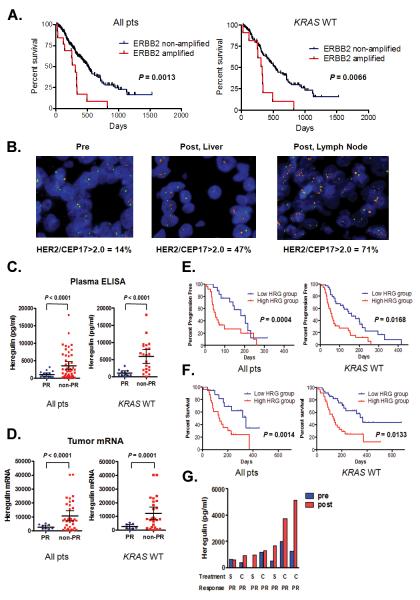
Figure 6. Both ERBB2 amplification and heregulin cause drug resistance in cetuximab treated colorectal cancer patients.
A. (Left) Overall survival for all CRC patients with (n = 13) and without ERBB2 amplification (n = 220) treated with cetuximab based therapy. Data for KRAS wild type only patients (ERBB2 amplified; n = 11; ERBB2 non-amplified; n = 171). Comparison based on log-rank test. B. ERBB2 FISH from a baseline primary tumor specimen (left) and following acquired cetuximab resistance in two independent drug resistant specimens (right). The patient was initially treated with single agent cetuximab and achieved a PR. ERBB2 (red) and CEP 17 (green). C. Scatter diagram of pre-treatment heregulin concentration in plasma from all (n = 65) or KRAS wild type only (n = 33) CRC patients achieving a PR and those not achieving a PR when treated with cetuximab based therapy. Mean ± 95% CI is shown. D. Scatter diagram of pre-treatment heregulin mRNA expression in tumors from all (n = 44) or KRAS wild type only (n = 34) CRC patients achieving a PR and those not achieving a PR when treated with cetuximab based therapy. Mean ± 95% CI is shown. E. (Left) Progression free survival for all CRC patients treated with cetuximab based therapy divided based on low (n = 35) or high (n = 35) plasma expression. (Right) Data for KRAS wild type only patients (low; n = 18; high n = 24). Comparison based on log-rank test. F. (Left) Overall survival for all CRC patients treated with cetuximab based therapy divided based on low (n = 35) or high (n = 35) plasma expression. (Right) Data for KRAS wild type only patients (low; n = 18; high n = 24). Comparison based on log-rank test. G. Comparisons of plasma levels of heregulin from CRC patients treated with cetuximab based therapy prior to therapy and after the development drug resistance. All patients achieved a PR. S; single agent cetuximab; C; combination with irinotecan.