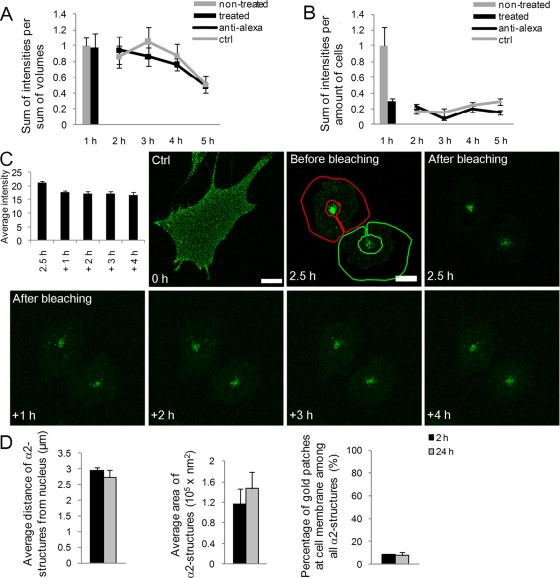
FIGURE 2:
Clustered α2 integrin is not recycled back to the plasma membrane. Recycling of (A) clustered and (B) unclustered α2 integrin was analyzed in SAOS-α2β1 cells by measuring the fluorescence intensity of surface-labeled integrin (Alexa 488) using repetitive treatments with the quenching anti–Alexa 488 antibodies. First, integrin was allowed to internalize for 1 h at 37°C and then treated with anti–Alexa 488 on ice for 30 min. Anti–Alexa 488 antibody treatment was thereafter repeated after 1-h intervals during subsequent incubations at 37°C for 4 h. Fluorescence intensity was measured from confocal z-stacks of images containing several cells. Altogether more than 50 cells from three independent experiments were analyzed. Results are shown as normalized mean values (±SE). (C) Recycling of α2 integrin from α2-MVBs back to plasma membrane was followed in living cells. Fab-DyLight 488–labeled α2 integrin was clustered with EV1 and internalized for 2.5 h. The edges of the cells were bleached, and trafficking of α2 integrin was observed at 1-h intervals. Average intensity of the bleached areas was quantified from three-dimensional sections. Bars, 10 μm. (D) The localization and size of α2 integrin–positive structures were determined from electron microscopy samples. First, anti–α2 integrin antibody was bound on cells on ice, followed by the clustering secondary antibody (rabbit anti-mouse) and protein A gold (10 nm). The gold clusters were allowed to internalize for 2 and 24 h. The size and location of gold particle positive structures were measured from 15 cells from two separate experiments (±SE) with iTEM software (Olympus). Altogether 75 and 108 vesicles were measured at 2 or 24 h, respectively.