Figure 10. Scheme summarizing effects of CK2 and CRN2 on actin dynamics.
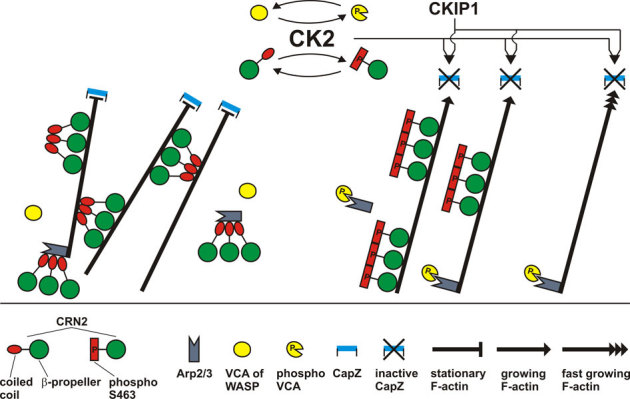
CK2 phosphorylates CRN2 at S463; CRN2 constitutively forms trimers independent of its phosphorylation state; CRN2 and phospho-CRN2 both bind to F-actin; CRN2 strongly inhibits actin polymerization, whereas phospho-CRN2 is nearly inactive; CRN2 strongly and phospho-CRN2 to a much lesser extent bundles F-actin; CRN2, but not phospho-CRN2 interacts with the Arp2/3 complex; both CRN2 and phospho-CRN2 compete with Arp2/3 for F-actin binding. Full-length CRN2 contains a large actin filament binding region formed by the β-propeller and a second distinct actin filament binding site within the coiled coil domain. Arp2/3 mediated branching of actin filaments is not included in this scheme. Data on the interactions of CK2 with VCA domain of WASP, and with CKIP-1 and CapZ were obtained from other studies50,51 and embedded into this scheme. Taken together, a picture emerges where CK2 acts in an integrative manner and promotes actin polymerization and suppresses actin filament bundling.
