Figure 2. CRN2 directly interacts with CK2α.
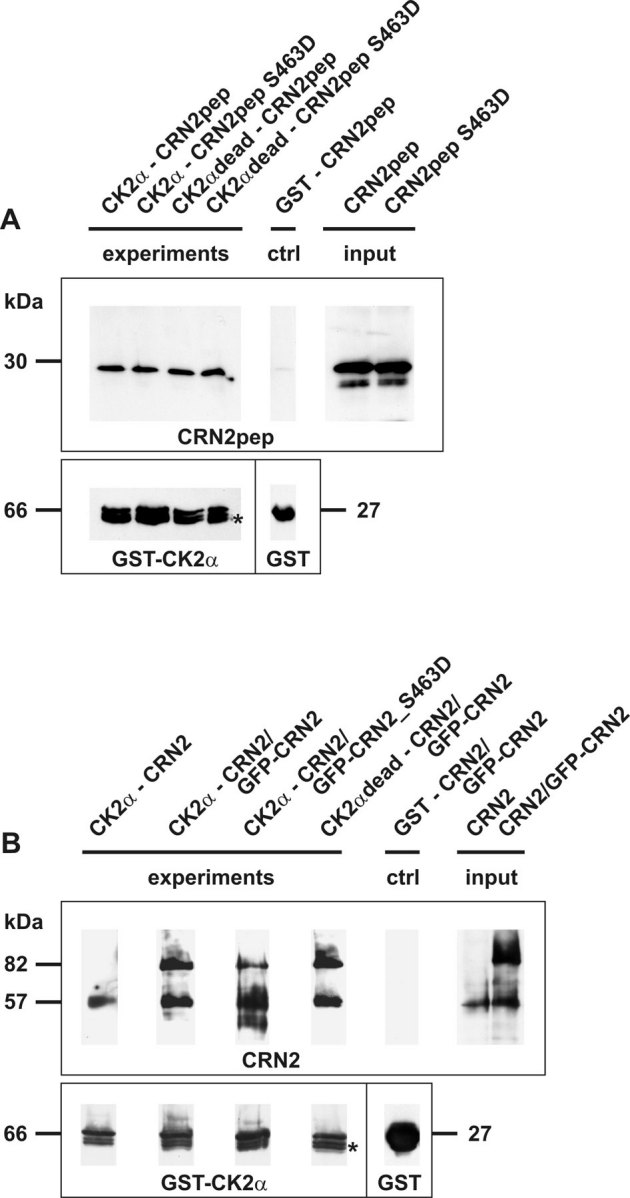
(A) pull-down assay employing purified recombinant full-length GST-tagged CK2α coupled to glutathione beads and purified soluble CRN2 wild-type and phosphomimetic S463D C-terminal polypeptides (aa300-474). Both polypeptides bind to CK2α and CK2αdead, the latter lacks kinase activity. (B) pull-down assay employing purified recombinant full-length GST-tagged CK2α coupled to glutathione beads and lysates from HEK293 cells over-expressing endogenous CRN2 (57 kDa) as well as GFP-CRN2 (82 kDa) fusion proteins. Endogenous, wild-type GFP-CRN2, and GFP-CRN2 S463D bind to CK2α. All CRN2 polypeptides were detected with antibody K6-444, and GST-CK2α immunoblotting was done with a rabbit polyclonal GST-antibody86. Asterisk, two additional CK2α bands are the result of degradation. Controls contained beads coated with GST alone. For illustration purposes individual lines from the original western blots were digitally re-arranged.
