Figure 3. Conformational Dynamics.
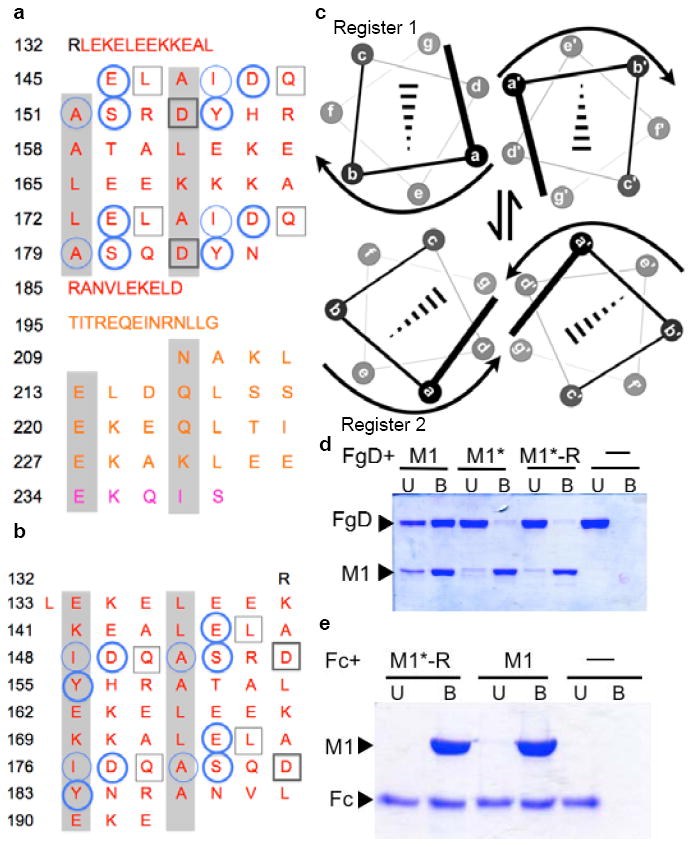
a, Heptad register of M1BC1 bound to FgD (register 2; a and d residues shaded gray). Residues not assigned a heptad position (132-144, 185-208) form an α-helical dimer but do not have coiled-coil “knobs-into-holes” packing. FgD-contacting residues are in circles for one M1 helix and in boxes for the other. Heavy lines denote polar contacts, and light lines vdW contacts. The B-repeats are in red, the S-region in gold, and C-repeat in purple. b, Heptad register of unbound M1AB (register 1; ref. 8). c, Relationship between registers 1 and 2. d, Association of His-tagged M1, M1*, and M1*-R with FgD at 37 °C, as assessed by a Ni2+-nitrilotriacetic acid (NTA) agarose coprecipitation assay and visualized by non-reducing, Coomassie-stained SDS-PAGE. U, unbound fraction; B, bound fraction. e, Association of His-tagged M1 and M1*-R with IgG Fc at 37 °C, as in panel d.
