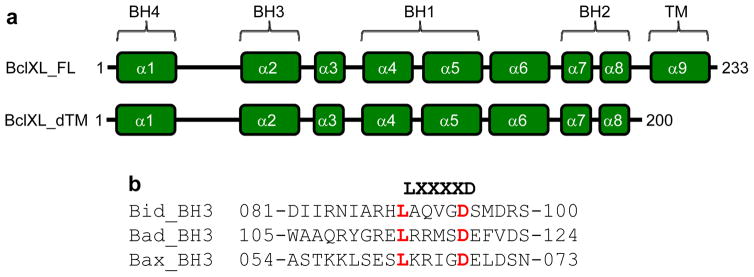
Figure 1.
BclXL domain organization and BH3 ligands. (a) Human BclXL is comprised of the BH4-BH3-BH1-BH2-TM modular organization, with a C-terminal transmembrane (TM) domain preceded by four N-terminal Bcl2 homology (BH) domains. The relationship between the various helices (α1–α9) punctuating the topological fold of BclXL and the BH domains is clearly indicated for clarity. While the BclXL_FL construct represents the full-length protein with the above modular organization, the C-terminal TM domain has been deleted in BclXL_dTM construct in order to investigate its function in the biological function of BclXL in this study. The numerals indicate amino acid boundaries within corresponding protein sequences. (b) Amino acid sequence alignment of 20-mer peptides spanning various BH3 domains within human Bid, Bad and Bax proteins. The numerals indicate amino acid boundaries within corresponding protein sequences. The LXXXXD motif characteristic of all BH3 domains is highlighted.