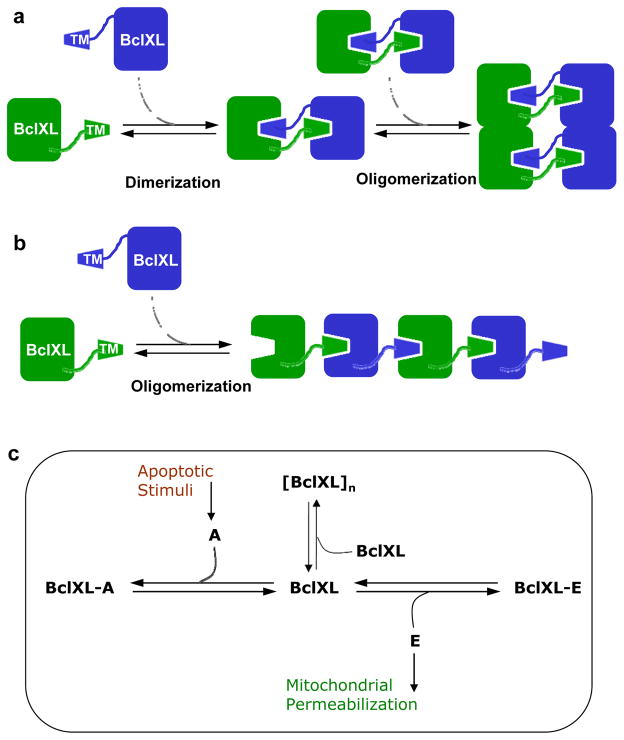
Figure 10.
Models for BclXL oligomerization and its role in apoptotic regulation. (a) Oligomerization of BclXL via a domain-swapped mechanism. The TM domain of one monomer (green) occupies the canonical hydrophobic groove within another monomer (blue) and vice versa to form a homodimer. The resulting homodimers, due to greater interacting molecular surface area, further self-associate into higher-order oligomers. (b) Oligomerization of BclXL via an inter-locking mechanism. The TM domain of one monomer (green) occupies the canonical hydrophobic groove within another monomer (blue) in a head-to-tail fashion so as to aid the assembly of much larger oligomers. (c) A thermodynamic cycle depicting how various linked-equilibria determine the fate of BclXL repressor to self-associate into higher-order [BclXL]n oligomers versus hetero-association with activator (A) and effector (E) molecules in quiescent versus apoptotic cells. In quiescent non-apoptotic cells, BclXL either self-associates into higher-order [BclXL]n oligomers and/or hetero-associates with effectors such as Bax and Bak, depending on the relative ratio of their cellular concentrations, to form BclXL-E repressor-effector complexes. In this manner, self-association into higher-order oligomers leads to inactivation of BclXL and hetero-association inactivates effectors. Upon receiving apoptotic stimuli, activators such as Bid and Bad compete with self-association of BclXL into higher-order oligomers and its hetero-association with effectors, leading to the formation of BclXL-A repressor-activator complexes as well as freeing up the effectors, which subsequently insert into MOM. This results in mitochondrial permeabilization leading to the release of apoptogenic factors that in turn induce cells to undergo apoptosis.