Abstract
During late stages of mammalian erythropoiesis the nucleus undergoes chromatin condensation, migration to the plasma membrane, and extrusion from the cytoplasm surrounded by a segment of plasma membrane. Since nuclear condensation occurs in all vertebrates, mammalian erythroid membrane and cytoskeleton proteins were implicated as playing important roles in mediating the movement and extrusion of the nucleus. Here we use erythroid ankyrin deficient and band 3 knockout mouse models to show that band 3, but not ankyrin, plays an important role in regulating the level of erythroid cell membrane proteins, as evidenced by decreased cell surface expression of glycophorin A in band 3 knockout mice. However, neither band 3 nor ankyrin are required for enucleation. These results demonstrate that mammalian erythroblast enucleation does not depend on the membrane integrity generated by the ankyrin-band 3 complex.
Keywords: Erythropoiesis, Enucleation, Ankyrin, Band 3, Glycoprotein
1. Introduction
Red blood cells are continuously replenished; in humans their half-life is 120 days. Erythropoietin (Epo), a cytokine produced by the kidney in response to low oxygen pressure, is the principal regulator of red blood cell production. Epo binds to cognate receptors on Colony Forming Unit Erythroid (CFU-E) progenitor cells and activates the JAK2 protein tyrosine kinase and several downstream signal transduction cascades including the Stat5, PI3K/Akt, and Ras-MAP kinase pathways [1]. These prevent CFU-E apoptosis and stimulate its terminal proliferation and differentiation; during the ensuing three days each CFU-E produces 30–50 erythroblasts that undergo terminal cell cycle exit, chromatin and nuclear condensation, and enucleation forming reticulocytes that are released into the circulation.
Multiple proteins and signaling pathways are involved in the enucleation process, including histone deacetylation, actin polymerization, cytokinesis, cell-matrix interactions, specific microRNAs, and vesicle trafficking [2,3]. Specifically, histone deacetylase 2 (HDAC2) is required for chromatin condensation and subsequent enucleation of mouse fetal liver erythroblasts[4]. Enucleation also requires Rac GTPases and their target formin mDia2 to form the contractile actin ring between the incipient reticulocyte and extruding nucleus [5].
Evidences from early studies indicated that enucleation is a process of cytokinesis where various proteins in membrane and cytoskeleton network are involved [6,7,8,9]. The organization and functions of this network have been well established. In brief, the heterodimeric α and β-spectrins self-associate into α2 β2 tetramers, which are connected by actins to form pentagonal or hexagonal lattices that underlie the plasma membrane [10]. The ankyrin-band 3 complex connects the spectrin-based skeleton to the plasma membrane and helps maintain the vertical and horizontal integrities of the mature erythrocyte. Mice with homozygous mutations of ankyrin (nb/nb mice) exhibit nearly complete ankyrin deficiency and severe hemolytic anemia [11]. The same phenotype is also observed in mice with a targeted depletion of band 3 [12]. On the other hand, mice carrying spontaneous mutations of α-spectrin (sph/sph mice) or β-spectrin (ja/ja mice) survive to birth but quickly die of severe hemolytic anemia [13]. In humans, functional disruption of these proteins frequently leads to hereditary spherocytosis (HS), hereditary elliptocytosis (HE) or hereditary pyropoikilocytosis (HPP) [14]. In light of the significance of the ankyrin-band 3 complex in the integrity of the red cell membrane, together with the fact that nuclear condensation occurs throughout the vertebrate phylum but that enucleation is only observed in mammals, we hypothesized that a deficiency of ankyrin or band 3 could affect the extent and/or rate of mammalian erythroblast enucleation.
Here we use our well-established in vitro mouse fetal liver erythroblasts culture system [5] to investigate enucleation in nb/nb and band 3 targeted knockout mice. We show that ankyrin and band 3 play distinct roles in the expression of erythroid cell surface protein glycophorin A, but that neither is required for the enucleation process. These results provide definitive evidence that the membrane-cytoskeleton integrity formed by the ankyrin-band 3 complex is not required for mammalian erythroblast enucleation.
2. Materials and Methods
2.1. Purification and culture of mouse fetal liver erythroblasts
Purification and culture of E13.5 TER119 negative mouse fetal liver erythroblasts were described previously [4,5,15]. Briefly, fetal liver cells were isolated from E13.5 embryos of different genotype and mechanically dissociated by pipetting in PBS containing 10% FBS (GIBCO). Single-cell suspensions were prepared by passing the dissociated cells through 70μm and 25μm cell strainers. Total fetal liver cells were labeled with biotin-conjugated anti-TER119 antibody (1:100) (BD Pharmingen), and TER119 negative cells were purified through a StemSep column according to the manufacturer’s instructions (Stem Cell Technologies). Purified cells were seeded in fibronectin-coated wells (BD Pharmingen) at a cell density of 1×105/ml and cultured in IMDM containing 15% FBS (StemCell Technologies), 1% detoxified bovine serum albumin (BSA), 200 μg/ml holo-transferrin (Sigma), 10 μg/ml recombinant human insulin (Sigma), 2 mM L-glutamine, 10−4 M β-mercaptoethanol, and 2 U/mL Epo (Amgen).
2.2. Flow cytometry analysis
Flow cytometric analysis of in vivo fetal liver erythroid cells and analysis of the differentiation status of cultured mouse fetal erythroblasts was described previously[5]. Briefly, fetal liver cells were immunostained with phycoerythrin (PE) conjugated anti-TER119 (1:200) (BD Pharmingen) and fluorescein isothiocyanate (FITC) conjugated anti-CD71 (1:200) (BD Pharmingen) antibodies, for 15 min at room temperature. Propidium iodide was added to exclude dead cells from analysis. For enucleation analysis, cells were stained with 10 μg/ml Hoechst 33342 (Sigma), in addition to fluorophore-conjugated antibodies, for 15 min at room temperature.
2.3. Statistical analysis
Statistical analysis was performed using GraphPad Prism. The student’s t-test was used to determine the significance of experimental groups versus control ones.
3. Results
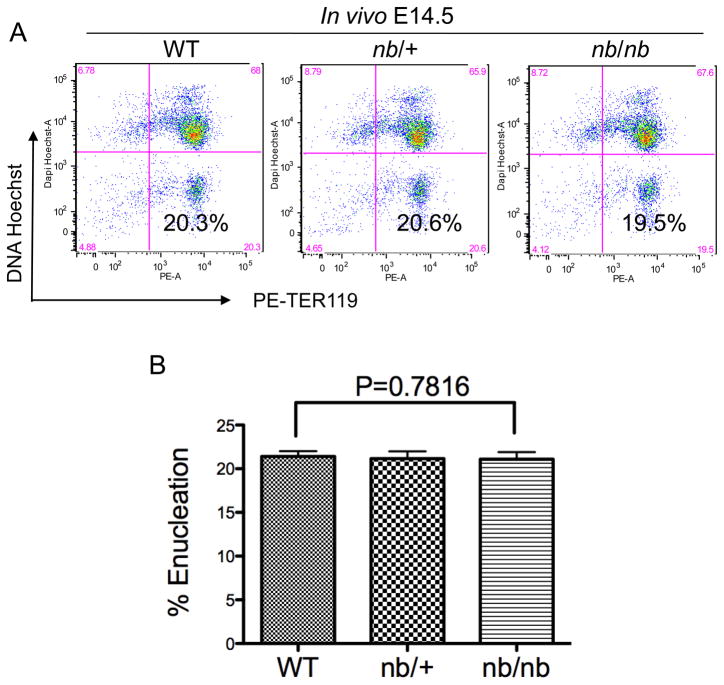
To directly investigate the roles of ankyrin in mammalian erythroblast enucleation, we first evaluated the extent of enucleation in vivo in nb/nb mice during fetal liver erythropoiesis, since more than 90% of the fetal liver cells are erythroid [5]. Specifically, total fetal liver cells from embryonic day 14.5 (E14.5) mice were purified and stained with erythroid-specific TER119 (antigen associated with erythroid cell-surface glycophorin A) and the cell permeable DNA staining dye Hoechst 33342 for flow cytometry analysis. Figure 1A shows that the expression levels of TER119 were essentially the same in wild type, nb/+ and nb/nb mice, which indicates that the absence of ankyrin does not influence the expression of a key red cell membrane protein. In addition, the percentages of enucleated cells (HoechstlowTER119high) exhibited no significant differences in these mice (Figure 1A and 1B).
Figure 1. Ankyrin deficiency does not affect cell surface TER119 expression or erythroblast enucleation in vivo.
(A) Flow cytometric analysis of mouse fetal erythroblast enucleation in vivo. The total population of fetal liver cells from E14.5 embryos in wild type (WT), nb/+, or nb/nb mice was stained with Hoechst 33342 and PE conjugated TER119 and analyzed by flow cytometry. Debris (low forward scatter) and dead cells (propidium iodide positive) were excluded from the analysis. The percentage of enucleated cells is indicated in the lower right quadrant. (B) Statistical analysis of enucleation rate in vivo in wild type, nb/+, and nb/nb mice.
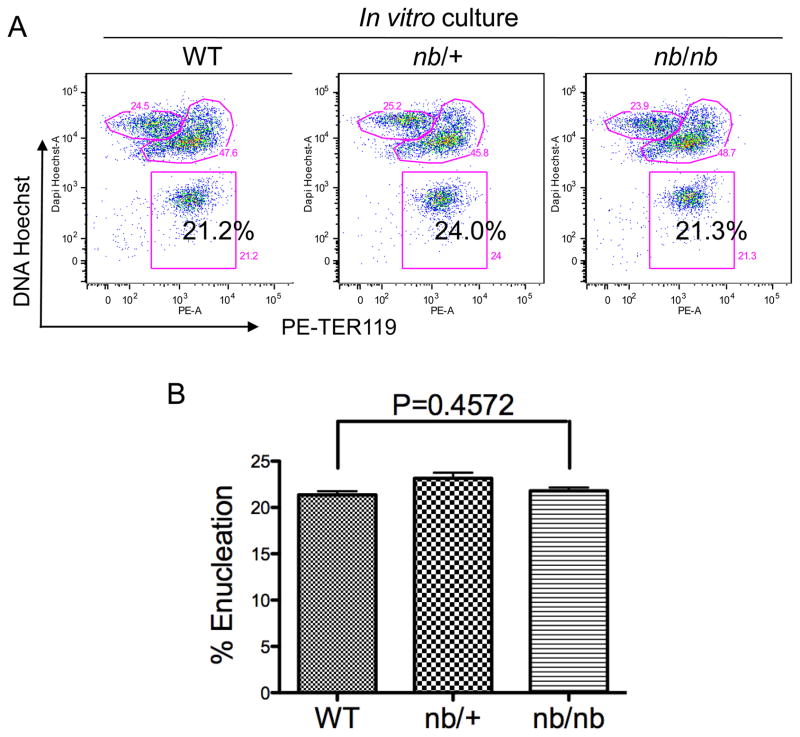
It is possible that stromal cells in the fetal liver compensate for the absence of ankyrin and facilitates the enucleation process. To circumvent this problem, we used our well-established in vitro erythroblast system in which purified E13.5 TER119 negative mouse fetal liver erythroblasts were cultured for 2 days on fibronectin- coated dishes in the presence of erythropoietin. The cells were harvested at day 2, stained with TER119 and Hoechst, and analyzed by flow cytometry. Figures 2A and 2B show that, as in vivo, loss of ankyrin did not affect the surface expression of TER119, or the extent of enucleation. These results demonstrate that ankyrin plays no significant function in enucleation of mammalian erythroblasts. These observations are also consistent with previously reported findings of no significant abnormalities of the red cell membrane or cytoskeleton in nb/nb mice [16].
Figure 2. Ankyrin deficiency does not affect cell surface TER119 expression or erythroblast enucleation in vitro.
(A) Analysis of mouse fetal erythroblast enucleation during in vitro culture. E13.5 fetal liver cells with the indicated genotype were stained with a biotinylated anti-TER119 monoclonal antibody. TER119 negative erythroid progenitor cells were purified and cultured in vitro in fibronectin-coated plates in medium containing serum and erythropoietin (Epo). The enucleation status of the cultured cells after two days of culture was analyzed by flow cytometric analysis using Hoechst 33342 and PE-TER-119. The percentage of enucleated cells is indicated. (B) Statistical analysis of the extent of enucleation in vitro in wild type, nb/+, and nb/nb mice.
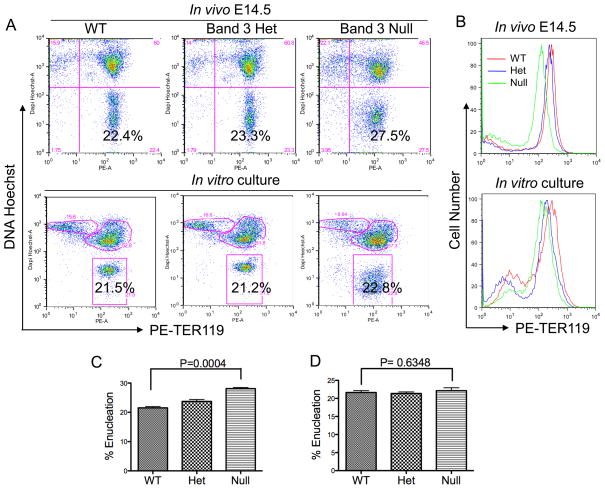
We next investigated the role of band 3 in enucleation. To this end, we used targeted band 3 knockout mice. Figures 3A and 3B show that in contrast to nb/nb mice, the cell surface expression level of glycophorin A (TER119) is decreased significantly – about three-fold - in band 3 knockout mice both in vivo and in erythroblasts formed during in vitro culture. In addition, the band 3 null erythroblasts also exhibited abnormal Hoechst staining, which could also be due to the disrupted membrane/cytoskeleton network.
Figure 3. Absence of Band 3 reduces TER119 expression on the erythroblast cell surface but does not affect enucleation.
(A) Flow cytometric analysis of mouse fetal erythroblast enucleation in vivo. The experiments were similar to those in Figure 1A (upper panels) and 2A (lower panels) except band 3 wild type (WT), heterozygous (Het), or knockout (null) mice were used. (B) Flow cytometric analysis of the surface TER119 expression levels of these mice in vivo (upper panel) and after in vitro culture (lower panel). Red, blue and green represent enucleated band 3 wild type (WT), heterozygous (Het) and knockout (null) erythroid cells, respectively. (C) Statistical analysis of enucleation extent in vivo in wild type, band 3 heterozygous and null mice. (D) Statistical analysis of enucleation extent in vitro in wild type, band 3 heterozygous and null mice.
Deficiency of band 3 led to a slight increase of enucleation in vivo (Figure 3A upper panels and Figure 3C) compared to wild type and heterozygous mice. However, there were no significant changes in the extent of enucleation when the TER119 negative fetal liver erythroblasts were cultured in vitro (Figure 3A lower panels and Figure 3D). The minor increase of enucleated cells in vivo in band 3 knockout fetal liver is likely a reactive reticulocytosis in response to the hemolytic anemia in the mouse, consistent with findings from previous report [12].
4. Discussion
In summary, our study provides definitive evidence that ankyrin and band 3 are not required for mouse fetal liver erythroblast enucleation. The hypothesis that mammalian erythroid membrane and cytoskeleton proteins are involved in enucleation is based on the fact that chromatin condensation occurs in all vertebrates but that only mammalian erythroblasts undergo nuclear extrusion[2,17]. During erythropoiesis, the membrane and cytoskeleton proteins undergo extensive reorganization. At the end of erythropoiesis, spectrin, actin, tubulin, ankyrin, glycophorin A and protein 4.1 partition to the incipient reticulocytes, whereas no major erythroid membrane or cytoskeleton protein are found in the extruded nuclei [8,18,19,20]. One possibility is that these reorganizations may facilitate the enucleation process, since morphologic studies showed that during late stages of erythropoiesis the nucleus moves to a patch of membrane that is deficient in the spectrin network [18]. It is still unclear what triggers the movement of the nucleus and how the nucleus “decides” where to migrate. One possibility is that the nucleus moves specifically to a segment of the plasma membrane lacking ankyrin and/or band 3. However, the present results make it unlikely that ankyrin or band 3 are involved in this migration process.
The ankyrin-band 3 complex plays critical roles in bridging spectrin/actin cytoskeleton to the cell surface lipid membrane and serving as a docking site for multiple membrane/cytoskeleton proteins. Although no significant numbers of circulating nucleated red blood cells are present in ankyrin- or spectrin- deficient mice [11,12], enucleation could still have been compromised in vitro since various compensatory pathways could have rescued the enucleation defect in vivo. Therefore, the absence of any effect on the extent or efficiency of enucleation in vitro in the absence of these proteins is a surprising finding.
Besides the ankyrin-band 3 complexes, other complexes also maintain the integrity of the red blood cell bilayer membrane. These complexes, such as that containing α and β adducin, are also unlikely to play any role in enucleation given the very mild phenotype of β adducin null mice [21]. In contrast to mice with defects in these complexes that bridge the spectrin cytoskeleton with the plasma membrane, mice carrying spontaneous mutations of α-spectrin (sph/sph mice) or β-spectrin (ja/ja mice) exhibit severe hemolytic anemia and die within weeks of birth [13]. It would therefore be interesting to investigate the role of spectrins in enucleation using these sph/sph and ja/ja mice.
Ankyrin and band 3 are not required for erythroblasts enucleation
Loss of ankyrin does not affect erythroid membrane glycoprotein expression
Loss of band 3 influences erythroid membrane glycoprotein expression
Acknowledgments
We thank Drs Luanne Peters and Sam Lux for nb/nb mice and band 3 knockout mice; Victor Yeh for the help with mice breeding. This study was supported by NIH grant K99HL102154 to P.J.; and NIH grant P01 HL 32262 and a research grant from Amgen, Inc. to H.F.L
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 2011;21:409–415. doi: 10.1016/j.tcb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keerthivasan G, Wickrema A, Crispino JD. Erythroblast enucleation. Stem Cells Int. 2011;2011:139851. doi: 10.4061/2011/139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji P, Yeh V, Ramirez T, Murata-Hori M, Lodish HF. Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica. 2010;95:2013–2021. doi: 10.3324/haematol.2010.029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol. 2008;10:314–321. doi: 10.1038/ncb1693. [DOI] [PubMed] [Google Scholar]
- 6.Simpson CF, Kling JM. The mechanism of denucleation in circulating erythroblasts. J Cell Biol. 1967;35:237–245. doi: 10.1083/jcb.35.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skutelsky E, Danon D. An electron microscopic study of nuclear elimination from the late erythroblast. J Cell Biol. 1967;33:625–635. doi: 10.1083/jcb.33.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koury ST, Koury MJ, Bondurant MC. Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J Cell Biol. 1989;109:3005–3013. doi: 10.1083/jcb.109.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chasis JA, Prenant M, Leung A, Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74:1112–1120. [PubMed] [Google Scholar]
- 10.Byers TJ, Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985;82:6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White RA, Birkenmeier CS, Lux SE, Barker JE. Ankyrin and the hemolytic anemia mutation, nb, map to mouse chromosome 8: presence of the nb allele is associated with a truncated erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990;87:3117–3121. doi: 10.1073/pnas.87.8.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters LL, Shivdasani RA, Liu SC, Hanspal M, John KM, Gonzalez JM, Brugnara C, Gwynn B, Mohandas N, Alper SL, Orkin SH, Lux SE. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 13.Kaysser TM, Wandersee NJ, Bronson RT, Barker JE. Thrombosis and secondary hemochromatosis play major roles in the pathogenesis of jaundiced and spherocytic mice, murine models for hereditary spherocytosis. Blood. 1997;90:4610–4619. [PubMed] [Google Scholar]
- 14.Tse WT, Lux SE. Red blood cell membrane disorders. Br J Haematol. 1999;104:2–13. doi: 10.1111/j.1365-2141.1999.01130.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 16.Yi SJ, Liu SC, Derick LH, Murray J, Barker JE, Cho MR, Palek J, Golan DE. Red cell membranes of ankyrin-deficient nb/nb mice lack band 3 tetramers but contain normal membrane skeletons. Biochemistry. 1997;36:9596–9604. doi: 10.1021/bi9704966. [DOI] [PubMed] [Google Scholar]
- 17.Orkin SH, Zon LI. Genetics of erythropoiesis: induced mutations in mice and zebrafish. Annu Rev Genet. 1997;31:33–60. doi: 10.1146/annurev.genet.31.1.33. [DOI] [PubMed] [Google Scholar]
- 18.Geiduschek JB, Singer SJ. Molecular changes in the membranes of mouse erythroid cells accompanying differentiation. Cell. 1979;16:149–163. doi: 10.1016/0092-8674(79)90196-x. [DOI] [PubMed] [Google Scholar]
- 19.Wickrema A, Koury ST, Dai CH, Krantz SB. Changes in cytoskeletal proteins and their mRNAs during maturation of human erythroid progenitor cells. J Cell Physiol. 1994;160:417–426. doi: 10.1002/jcp.1041600304. [DOI] [PubMed] [Google Scholar]
- 20.Lee JC, Gimm JA, Lo AJ, Koury MJ, Krauss SW, Mohandas N, Chasis JA. Mechanism of protein sorting during erythroblast enucleation: role of cytoskeletal connectivity. Blood. 2004;103:1912–1919. doi: 10.1182/blood-2003-03-0928. [DOI] [PubMed] [Google Scholar]
- 21.Muro AF, Marro ML, Gajovic S, Porro F, Luzzatto L, Baralle FE. Mild spherocytic hereditary elliptocytosis and altered levels of alpha- and gamma-adducins in beta-adducin-deficient mice. Blood. 2000;95:3978–3985. [PubMed] [Google Scholar]