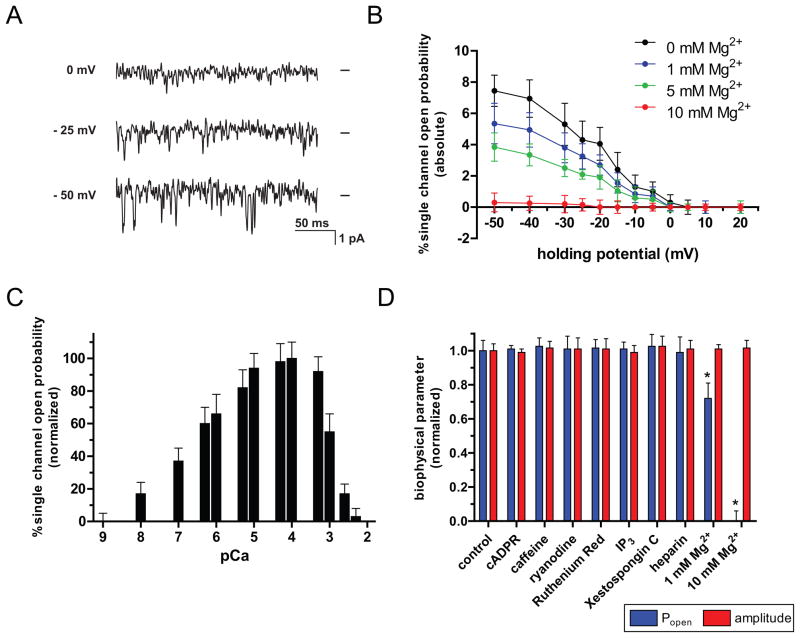
Figure 4. Biophysical and pharmacological characteristics of single channel polycystin-2 isolated from RGCs.
(A) Single channel currents of RGC polycystin-2 were recorded at different holding potentials. Representative traces of a typical experiment with native channels isolated from ER microsomes and incorporated into bilayer lipid membranes are shown. Single channel activity was recorded at a cytoplasmic Ca2+ concentration of 0.1 μM. Zero current levels are indicated by bars at the right of each trace and downward deflections indicate channel openings. Channels display a pronounced voltage-dependence and activation by negative holding potentials. (B) The single-channel open probability of native channels isolated from RGC ER microsomes was measured in the presence of 0.1 μM cytoplasmic Ca2+ as a function of the membrane holding potential. Addition of cytosolic Mg2+ reduced single-channel open probability in a dose-dependent manner. Data is shown as mean ± s.e.m. (n=4). (C) The normalized single-channel open probability of native polycystin-2 isolated from RGC ER microsomes was measured in the presence of different cytosolic Ca2+ concentrations plotted as pCa. Both increased single-channel mean open time and opening frequency contributed to elevated single-channel open probability in the physiological range of cytosolic Ca2+ concentrations. Data is shown as mean ± s.e.m. (n=4). (D) Dependence of biophysical characteristics of single channel polycystin-2 isolated from RGCs on pharmacological modulators of intracellular calcium channels. Properties of native channels isolated from RGC ER microsomes were measured in the presence of 0.1 μM cytoplasmic Ca2+ at a sub-maximal holding potential of −10mV. Compounds were added to the cytoplasmic side of the channel at concentrations that had been established previously to modulate other intracellular Ca2+ channels (cADPR: 10 μM; caffeine: 10 mM; ryanodine: 10 μM; ruthenium red: 10 μM; IP3: 1 μM, Xestospongine C: 1 μM; heparin: 50 μg/ml) and single-channel open probability and amplitude were monitored. Neither one of the two biophysical parameters were affected by these compounds. Addition of cytosolic Mg2+, however, reduced channel activity significantly at 1 mM and blocked activity a cytosolic concentration of 10 mM while single-channel amplitude was not affected by cytosolic Mg2+. Data is shown as mean ± s.e.m. (n=4). * P<0.05.