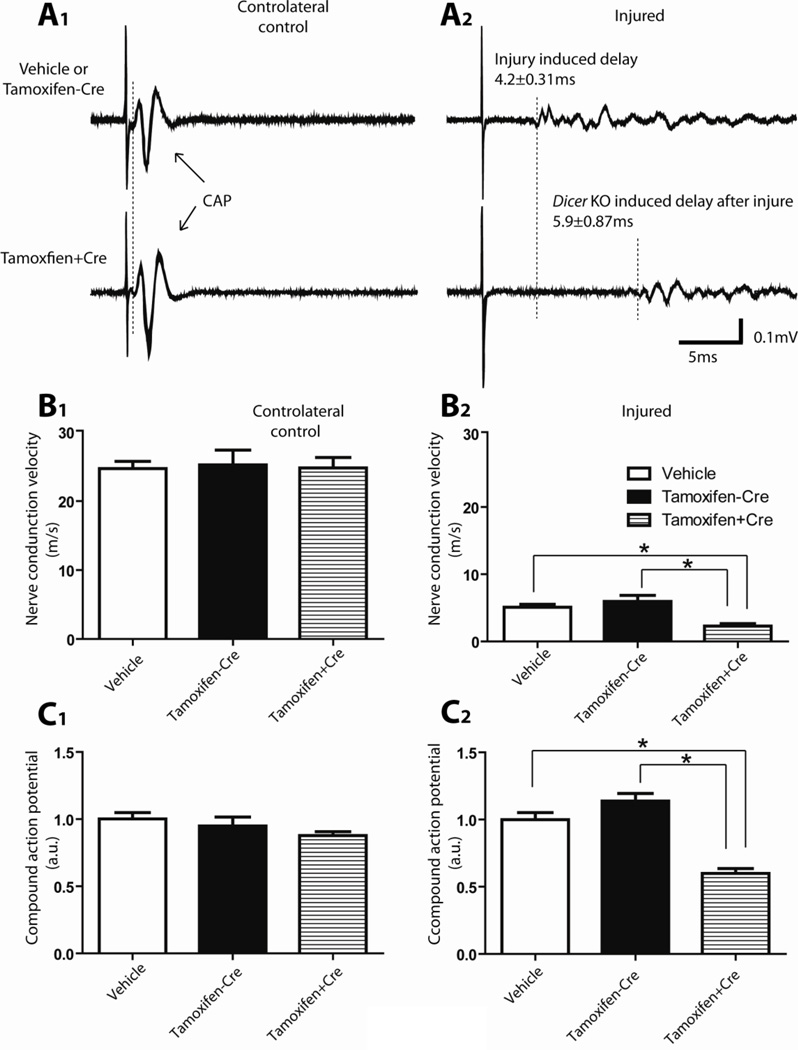
Figure 3. Electrophysiological evaluations of sciatic nerve functional recovery at 14 days after sciatic nerve crush.
A, Typical examples of responses recorded from the distal side of the sciatic nerve after electrical stimulation. Stimulation of the proximal side of the sciatic nerves (500 µA, 100 µsec) induced a fast response with a single CAP in the intact nerve and a delayed and spread-out response in the regenerating nerve. A1, Compound action potentials (CAPs) recorded from the controlateral uninjured sciatic nerve. The signals recorded from vehicle treated mice and tamoxifen treated Dicerfl/fl mice (no Dicer deletion) were similar to that recorded from tamoxifen treated CAG-CreERt:Dicerfl/fl mice (Dicer KO). The duration from the stimulation artifact to onset of compound action potential was used for the calculation of nerve conduction velocity. No difference of NCV (B1) or rectified and integrated compound action potential amplitude (C1) was observed between the different groups in the controlateral uninjured nerves. A2, Delayed responses were recorded from the injured sciatic nerves 14 days after crush. Note the additional delay in the response of Dicer KO sciatic nerve of nearly 6 ms, indicating a reduced nerve conduction velocity, and suggesting a slower rate of functional recovery after injury in the Dicer KO animals. B2, Animals from all 3 groups gradually restored NCV on the crushed nerve with time. However, animals with Dicer KO (tamoxifen treated CAG-CreERt:Dicerfl/fl) have a significantly lower NCV compared with control animals (tamoxifen treated Dicerfl/fl mice and vehicle treated mice). C2, Dicer KO animals also exhibited smaller rectified and integrated response amplitude (N=5, * p<0.05).