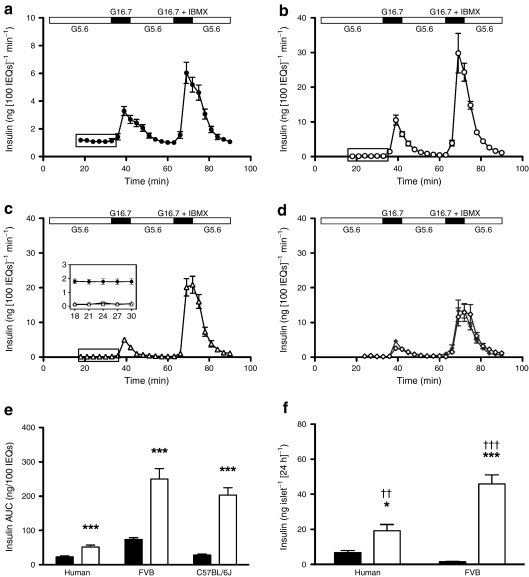
Fig. 3.
Insulin secretion in human and mouse islets. a Dynamic glucose-regulated insulin secretory characteristics of perifused human islets (n = 24), (b) FVB mouse islets (n = 9), (c) C57BL/6J mouse islets (n = 8) and (d) shipped C57BL/6J islets. The insulin concentration was determined by radioimmunoassay [19]. Note that the scale for the y-axis is different for human islets to make the insulin secretory pattern more readily visible. The amount of insulin secreted was normalised to IEQ, but it should be realised that islet cell composition in humans is different to that in mice [3]. Based on our experience with mouse and human islet preparations, our rationale for normalising insulin to IEQ is described above (Methods). ESM Fig. 3 shows secreted insulin normalised to islet insulin content. Here (a–d), the isolated human and mouse islets were precultured in 5 mmol/l glucose for 72 and 48 h, respectively. Human islets (a) showed a higher insulin output at basal 5.6 mmol/l glucose (G5.6) than mouse islets (b–d), and a lower stimulation index at 16.7 mmol/l glucose (G16.7) or 16.7 mmol/l glucose + 100 μmol/l IBMX (G16.7 + IBMX). The insert (c) shows basal secretion by human (black symbols), and FVB and C57BL/6J (white symbols) islets, which in the latter was similar and appears to overlap. An additional 24 h of culture of mouse islets did not change the magnitude of the perifusion response; dynamic GSIS in mouse islets was similar at 48 and 72 h of culture (data not shown). d To assess the effects of shipping on islet function, C57BL/6J mouse islets were isolated at Vanderbilt (n = 5 islet isolations; three separate shipments) and cultured for 24 h. Identical islet aliquots were either cultured in RPMI + 10% FBS (5 mmol/l glucose) or shipped by overnight courier to the University of Massachusetts using the shipping containers and conditions used by the Islet Cell Resource Centers and the Integrated Islet Distribution Network. Upon arrival at the University of Massachusetts, the shipping container was opened, labelled with a new shipping label and shipped back by the same overnight courier. Upon arrival at Vanderbilt, the cultured (black diamonds) and shipped (white diamonds) islet aliquots were simultaneously evaluated in the perifusion system (i.e. at approximately 72 h after isolation, with approximately 48 h of shipping time). Significantly, control C57BL/6J islets (cultured for approximately 72 h) and shipped islets had similar insulin secretory properties, as assessed by AUC per 100 IEQ; p = 0.368 for 16.7 mmol/l glucose peak; p = 0.322 for glucose + IBMX peak. e Integrated insulin secretion per 100 IEQ for mouse and human islets after stimulation with 16.7 mmol/l glucose (black bars) or 16.7 mmol/l glucose + 100 μmol/l IBMX (white bars); ***p < 0.001 for comparison with 16.7 mmol/l. One-way ANOVA was used for multiple group comparisons, with p values as follows: 16.7 mmol/l p < 0.001 for human vs FVB, p > 0.05 for human vs C57 and p < 0.001 for FVB vs C57; 16.7 mmol/l glucose + 100 μmol/l IBMX p < 0.001 for human vs FVB and human vs C57, and p < 0.01 for FVB vs C57. f Static GSIS was measured in human (n = 8) and FVB mouse islets (n = 8) exposed to 5 (black bars) or 11 mmol/l (white bars) glucose for an additional 24 h. Human islets showed significantly higher basal insulin secretion than mouse, whereas GSIS in human islets increased only three fold vs 30-fold in mouse islets; *p < 0.05 and ***p < 0.001 for comparison with 5 mmol/l glucose; †† p < 0.01 and ††† p < 0.001 for comparison with human islets