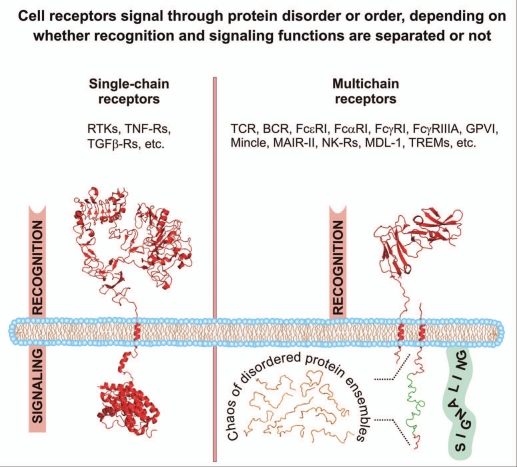
Figure 1.
Intrinsic order and disorder of the cytoplasmic signaling (effector) domains of single-and multichain cell receptors. Images were created using PyMol (www.pymol.org) from Protein Data Bank entries 1NQL and 3GOP for the EGFR extracellular domain, juxtamembrane and kinase domains, respectively (shown as an exemplary structure of a single-chain receptor) and entry 1UCT for the FcαRI extracellular domain (shown as an exemplary structure of a multichain receptor recognition subunit). For illustrative purposes, the cytoplasmic domain of a multichain receptor-associated signaling subunit is shown as a monomer and using arbitrary idealized structural elements to represent the ensemble of unfolded conformations of an IDR. The immunoreceptor tyrosine-based activation motif (ITAM) is depicted in green. Abbreviations: BCR, B cell receptor; DAP-12, DNAX adapter protein of 12 kD; FcαRI, type I Fc receptor for IgA; FcγRI, type I Fc receptor for IgG; FcεRI, type I Fc receptor for IgE; FcγRIIIA, type IIIA Fc receptor for IgG; GPVI, glycoprotein VI; IDR, intrinsically disordered region; MAIR-II, myeloid-associated Ig-like receptor; MDL-1, myeloid DAP12-associating lectin 1; Mincle, a C-type lectin receptor expressed in activated phagocytes; NK-R, natural killer cell receptor; RTK, receptor tyrosine kinase; TCR, T cell receptor; TGFβ-R, transforming growth factor-beta receptor; TNF-R, tumor necrosis factor receptor; TREM, triggering receptor expressed on myeloid cells.