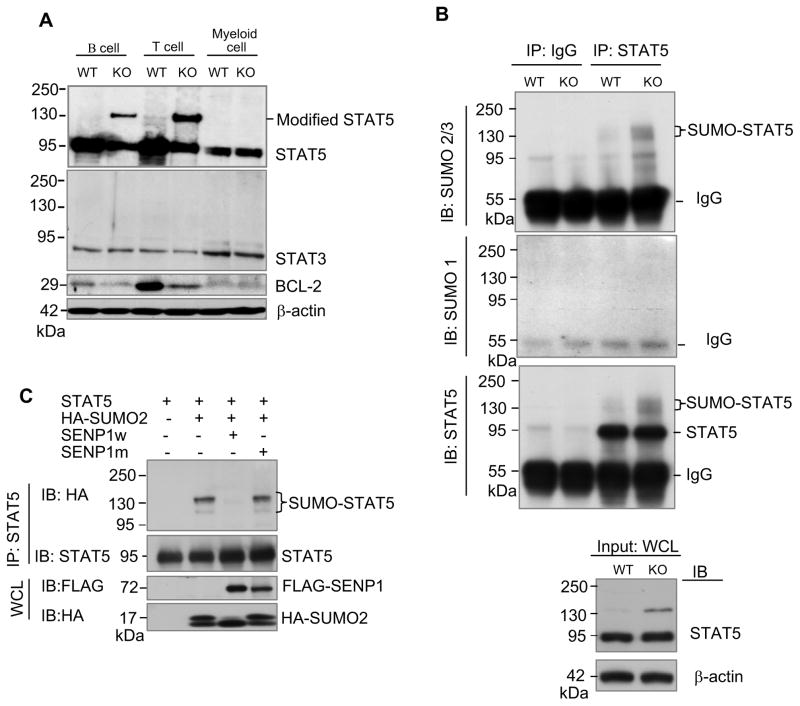
Figure 4. SENP1 regulates SUMOylation of STAT5.
(A) Modified STAT5 accumulated in SENP1−/− B and T cells. Sorted FL-HSCs from wild-type and SENP1−/− embryos at E14.5 were cocultured on OP9 and OP9-DL1 cells to differentiate into B and T cells, respectively. After 7 days of coculture, B and T cells were isolated and lysed in RIPA buffer. The whole cell lysates were analyzed by immunobloting with anti-STAT5 (top panel), anti-STAT3 (second panel), anti-BCL-2 (third panel), and anti-β-actin (bottom) antibodies. Wild-type and SENP1−/− myeloid cells derived from HSC differentiation on OP9 stromal cells for 7 days were used as controls. The results shown are a representative of at least three independent experiments.
(B) Endogenous STAT5 is SUMOylated by endogenous SUMO2/3 in vivo. B cells isolated from differentiation of wild-type and SENP1−/− FL-HSCs after 7 days of coculture on OP9 cells were lysed and boiled in denaturing lysis buffer. Cell lysates were aliquoted equally for performing immunoprecipitation (IP) by control IgG or a mixture of anti-STAT5A and B antibodies. Bound proteins were detected by immunoblotting (IB) with anti-SUMO2/3 (top panel), anti-SUMO1 (middle panel) or anti-STAT5 (bottom panel) antibodies. Loading samples were immunoblotted with anti-STAT5 or anti-β actin antibodies. The results shown are a representative of at least three independent experiments.
(C) SENP1 de-SUMOylates SUMOylated STAT5 in vivo. COS-1 cells were transfected with the indicated plasmids. Protein extracts were precipitated with anti-STAT5B antibody and analyzed with HA (top) and STAT5 (bottom) antibodies. The results shown are a representative of at least three independent experiments.