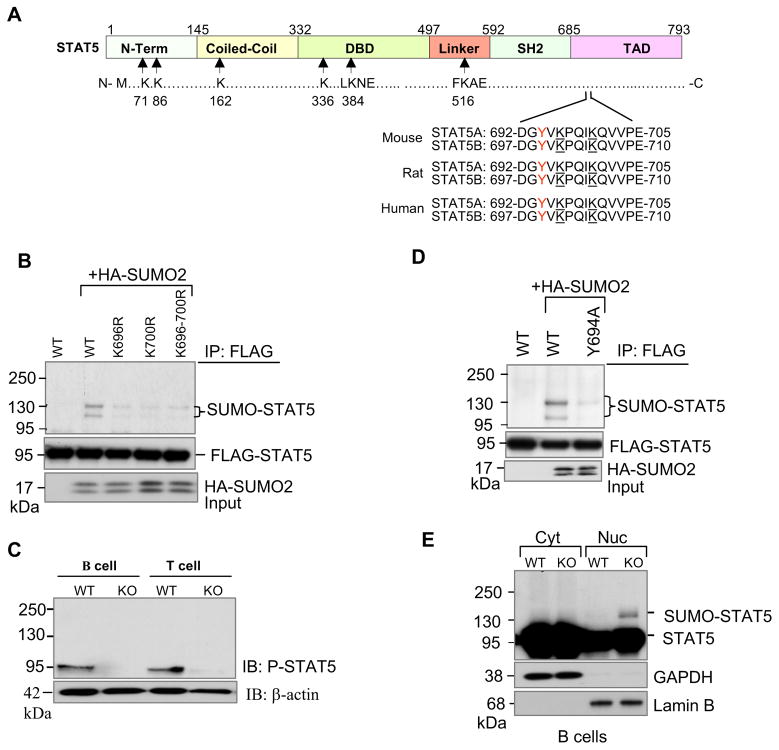
Figure 5. SUMOylation inhibits tyrosine phosphorylation of STAT5.
(A) Schematic diagram of full-length murine STAT5 protein structure. STAT5 contains amino terminal, coiled-coil, DNA binding, linker, SH2, and transcriptional activation domains. Arrows indicate approximate locations of two putative SUMO consensus motifs at lysines 384 and 516, and four other non-consensus motifs at lysines 71, 86, 163 and 336. Two lysine residues 696 and 700 located close to Y694 and Y699 of STAT5A and STAT5B respectively are underlined.
(B) STAT5A is modified by SUMO at lysine residues 696 and 700. COS-1 cells were transfected with FLAG-tagged STAT5A and its mutants along with HA-SUMO2 plasmids. Protein extracts were immunoprecipitated with anti-FLAG and then revealed by Western blot analysis with antibodies to anti-HA (top) and anti-FLAG (bottom). The results shown are representative of at least three independent experiments.
(C) Tyrosine phosphorylation of STAT5 is diminished in SENP1−/− B and T cells. Sorted FL-HSCs from wild-type and SENP1−/− embryos at E14.5 were cocultured on OP9 and OP9-DL1 cells in the presence of IL-7 and Flt3L to differentiate into B and T cells, respectively. After 7 days of coculture, B and T cells were isolated and lysed in RIPA buffer. The whole cell lysates (prepared and analyzed in Fig. 4A) were further analyzed by immunobloting with anti-P-STAT5 (top panel) and anti-β-actin (bottom) antibodies. The results shown are a representative of at least three independent experiments.
(D) Phosphorylation is required for STAT5 SUMOylation. COS-1 cells were transfected with FLAG-tagged wild-type STAT5A, or STAT5A phosphorylation mutant Y694A (mutation of tyrosine 694 to alanine), and HA-tagged SUMO-2 plasmids. Twenty four hours after transfection, cellular protein extracts were immunoprecipitated with anti-FLAG antibody followed by Western blot analysis with anti-HA (top panel) or anti-FLAG antibodies (second panel). Cell lysates were analyzed by immunoblotting with anti-HA antibody (bottom panel). The results are a representative of at least three independent experiments.
(E) SUMOylated forms of STAT5 were localized in the nucleus. Cytoplasmic (Cyt) and nuclear (Nuc) fractions from wild-type and SENP1−/− B cells harvested from OP9 coculture at day 7 were prepared, followed by Western blot analysis with anti- STAT5 antibody (top panel), and fraction purity was determined using antibodies against GAPDH (cytoplasmic, second panel) and Lamin B (nuclear, bottom panel).