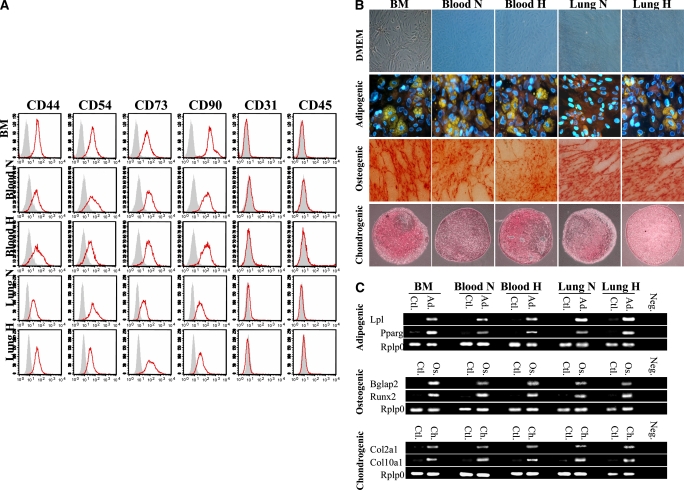
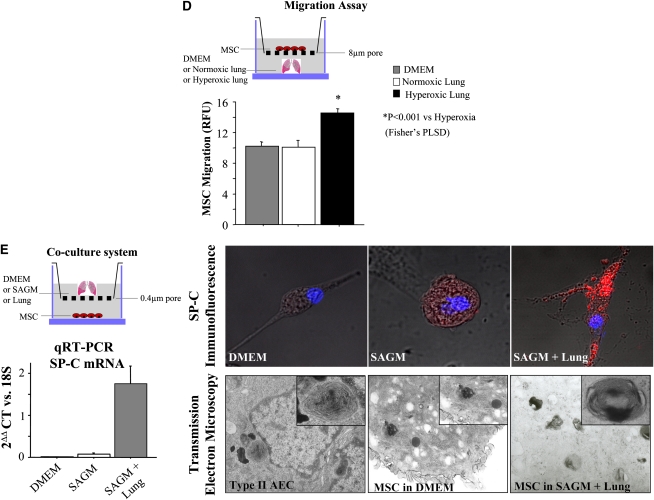
Figure 1.
(A) Characterization of mesenchymal stem cell (MSC) immunophenotype. Shown are fluorescence intensity histograms with specific antibodies for membrane antigens (red line) and irrelevant isotypic-matched antibody as negative control (gray area). Experiments were performed in triplicate. MSC differentiation potential was assessed by (B) histochemistry and (C) mRNA expression of lineage-specific genes for adipogenic (Ad), osteogenic (Os), and chondrogenic (Ch) differentiation. Passage 2 bone marrow (BM)–derived adherent cells, after culture in differentiation medium (Ad, Os, or Ch), were stained for oil red O (Ad staining), alizarin red (Os staining), and safranin O (Ch staining). Ctl = control proliferation medium; Neg = polymerase chain reaction without cDNA. All experiments were performed in triplicate. DMEM = Dulbecco's modified Eagle's medium; H = hypoxic; N = normoxic. (D) Migration assay in Boyden chamber. In vitro, MSCs placed in the upper chamber migrate preferentially to lungs from O2-exposed rats than to culture medium only or lungs from rats housed in room air. PLSD = Fisher's probable least significant difference; RFU = relative fluorescence units. (E) In vitro, bone marrow–derived mesenchymal stem cells (BMSCs) differentiate into type II alveolar epithelial cells (AEC2). The lung microenvironment induces BMSCs to adopt an AEC2 phenotype. Co-culture experiments of BMSCs with O2-injured lung, but not with culture medium alone, show molecular (surfactant protein [SP]-C mRNA and protein expression) and ultrastructural (lamellar bodies in electron microscopy) features of AEC2. SAGM = small airway growth medium.